Hollow Particles Obtained by Prilling and Supercritical Drying as a Potential Conformable Dressing for Chronic Wounds
- PMID: 37367162
- PMCID: PMC10298422
- DOI: 10.3390/gels9060492
Hollow Particles Obtained by Prilling and Supercritical Drying as a Potential Conformable Dressing for Chronic Wounds
Abstract
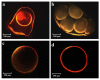
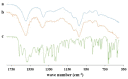
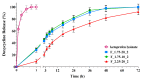
The production of aerogels for different applications has been widely known, but the use of polysaccharide-based aerogels for pharmaceutical applications, specifically as drug carriers for wound healing, is being recently explored. The main focus of this work is the production and characterization of drug-loaded aerogel capsules through prilling in tandem with supercritical extraction. In particular, drug-loaded particles were produced by a recently developed inverse gelation method through prilling in a coaxial configuration. Particles were loaded with ketoprofen lysinate, which was used as a model drug. The core-shell particles manufactured by prilling were subjected to a supercritical drying process with CO2 that led to capsules formed by a wide hollow cavity and a tunable thin aerogel layer (40 μm) made of alginate, which presented good textural properties in terms of porosity (89.9% and 95.3%) and a surface area up to 417.0 m2/g. Such properties allowed the hollow aerogel particles to absorb a high amount of wound fluid moving very quickly (less than 30 s) into a conformable hydrogel in the wound cavity, prolonging drug release (till 72 h) due to the in situ formed hydrogel that acted as a barrier to drug diffusion.
Keywords: aerogel capsules; ketoprofen lysine; prilling; supercritical drying; wound dressing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rezvani Ghomi E., Khalili S., Nouri Khorasani S., Esmaeely Neisiany R., Ramakrishna S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019;136:47738. doi: 10.1002/app.47738. - DOI
LinkOut - more resources
Full Text Sources

