Cryopreservation of 3D Bioprinted Scaffolds with Temperature-Controlled-Cryoprinting
- PMID: 37367172
- PMCID: PMC10298045
- DOI: 10.3390/gels9060502
Cryopreservation of 3D Bioprinted Scaffolds with Temperature-Controlled-Cryoprinting
Abstract
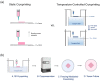
Temperature-Controlled-Cryoprinting (TCC) is a new 3D bioprinting technology that allows for the fabrication and cryopreservation of complex and large cell-laden scaffolds. During TCC, bioink is deposited on a freezing plate that descends further into a cooling bath, keeping the temperature at the nozzle constant. To demonstrate the effectiveness of TCC, we used it to fabricate and cryopreserve cell-laden 3D alginate-based scaffolds with high cell viability and no size limitations. Our results show that Vero cells in a 3D TCC bioprinted scaffold can survive cryopreservation with a viability of 71%, and cell viability does not decrease as higher layers are printed. In contrast, previous methods had either low cell viability or decreasing efficacy for tall or thick scaffolds. We used an optimal temperature profile for freezing during 3D printing using the two-step interrupted cryopreservation method and evaluated drops in cell viability during the various stages of TCC. Our findings suggest that TCC has significant potential for advancing 3D cell culture and tissue engineering.
Keywords: 3D bioprinting; 3D cell culture; alginate; cryopreservation; freezing.
Conflict of interest statement
Boris Rubinsky has patent #US2022016827A1 issued to THE REGENTS OF THE UNIVERSITY OF CALIFORNIA.
Figures




References
-
- Pegg D.E. Principles of Cryopreservation. In: Wolkers W., Oldenhof H., editors. Cryopreservation and Freeze-Drying Protocols. Volume 1257. Humana Press Inc.; Totowa, NJ, USA: 2015. pp. 3–19.

