Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering
- PMID: 37367175
- PMCID: PMC10298285
- DOI: 10.3390/gels9060505
Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering
Abstract
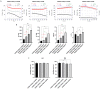
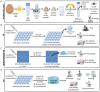
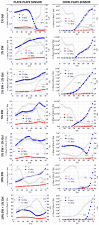
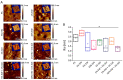
Innovative materials are needed to produce scaffolds for various tissue engineering and regenerative medicine (TERM) applications, including tissue models. Materials derived from natural sources that offer low production costs, easy availability, and high bioactivity are highly preferred. Chicken egg white (EW) is an overlooked protein-based material. Whilst its combination with the biopolymer gelatin has been investigated in the food technology industry, mixed hydrocolloids of EW and gelatin have not been reported in TERM. This paper investigates these hydrocolloids as a suitable platform for hydrogel-based tissue engineering, including 2D coating films, miniaturized 3D hydrogels in microfluidic devices, and 3D hydrogel scaffolds. Rheological assessment of the hydrocolloid solutions suggested that temperature and EW concentration can be used to fine-tune the viscosity of the ensuing gels. Fabricated thin 2D hydrocolloid films presented globular nano-topography and in vitro cell work showed that the mixed hydrocolloids had increased cell growth compared with EW films. Results showed that hydrocolloids of EW and gelatin can be used for creating a 3D hydrogel environment for cell studies inside microfluidic devices. Finally, 3D hydrogel scaffolds were fabricated by sequential temperature-dependent gelation followed by chemical cross-linking of the polymeric network of the hydrogel for added mechanical strength and stability. These 3D hydrogel scaffolds displayed pores, lamellae, globular nano-topography, tunable mechanical properties, high affinity for water, and cell proliferation and penetration properties. In conclusion, the large range of properties and characteristics of these materials provide a strong potential for a large variety of TERM applications, including cancer models, organoid growth, compatibility with bioprinting, or implantable devices.
Keywords: egg white; gelatin; hydrocolloids; hydrogels; microfluidics; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Boron nitride nanotubes reinforced gelatin hydrogel-based ink for bioprinting and tissue engineering applications.Biomater Adv. 2022 Oct;141:213103. doi: 10.1016/j.bioadv.2022.213103. Epub 2022 Sep 2. Biomater Adv. 2022. PMID: 36084352
-
The Impact of Gelatin and Fish Collagen on Alginate Hydrogel Properties: A Comparative Study.Gels. 2024 Jul 25;10(8):491. doi: 10.3390/gels10080491. Gels. 2024. PMID: 39195020 Free PMC article.
-
Highly Organized Porous Gelatin-Based Scaffold by Microfluidic 3D-Foaming Technology and Dynamic Culture for Cartilage Tissue Engineering.Int J Mol Sci. 2022 Jul 30;23(15):8449. doi: 10.3390/ijms23158449. Int J Mol Sci. 2022. PMID: 35955581 Free PMC article.
-
Recent Advances on Bioprinted Gelatin Methacrylate-Based Hydrogels for Tissue Repair.Tissue Eng Part A. 2021 Jun;27(11-12):679-702. doi: 10.1089/ten.TEA.2020.0350. Epub 2021 Mar 9. Tissue Eng Part A. 2021. PMID: 33499750 Review.
-
Exploiting the role of nanoparticles for use in hydrogel-based bioprinting applications: concept, design, and recent advances.Biomater Sci. 2021 Sep 28;9(19):6337-6354. doi: 10.1039/d1bm00605c. Biomater Sci. 2021. PMID: 34397056 Review.
Cited by
-
Construction and Evaluation of Alginate Dialdehyde Grafted RGD Derivatives/Polyvinyl Alcohol/Cellulose Nanocrystals IPN Composite Hydrogels.Molecules. 2023 Sep 19;28(18):6692. doi: 10.3390/molecules28186692. Molecules. 2023. PMID: 37764467 Free PMC article.
-
Revealing the Control Mechanisms of pH on the Solution Properties of Chitin via Single-Molecule Studies.Molecules. 2023 Sep 22;28(19):6769. doi: 10.3390/molecules28196769. Molecules. 2023. PMID: 37836611 Free PMC article.
-
A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies.Nanomaterials (Basel). 2023 Sep 18;13(18):2585. doi: 10.3390/nano13182585. Nanomaterials (Basel). 2023. PMID: 37764614 Free PMC article. Review.
-
Compound Microalgae-Type Biofunctional Hydrogel for Wound Repair during Full-Thickness Skin Injuries.Polymers (Basel). 2024 Mar 3;16(5):692. doi: 10.3390/polym16050692. Polymers (Basel). 2024. PMID: 38475375 Free PMC article.
-
Designing Composite Stimuli-Responsive Hydrogels for Wound Healing Applications: The State-of-the-Art and Recent Discoveries.Materials (Basel). 2024 Jan 5;17(2):278. doi: 10.3390/ma17020278. Materials (Basel). 2024. PMID: 38255446 Free PMC article. Review.
References
-
- García-Gareta E. Collagen, from Tissue Culture to Biomaterials, Tissue Engineering, and Beyond. 1st ed. Cambridge Scholars Publishing; Newcastle upon Tyne, UK: 2019.
Grants and funding
LinkOut - more resources
Full Text Sources

