Analysis of the Ability of Different Allografts to Act as Carrier Grafts for Local Drug Delivery
- PMID: 37367268
- PMCID: PMC10299409
- DOI: 10.3390/jfb14060305
Analysis of the Ability of Different Allografts to Act as Carrier Grafts for Local Drug Delivery
Abstract
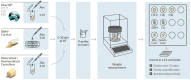
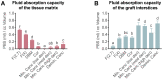
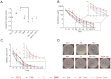
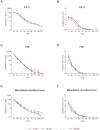
Bone defects and infections pose significant challenges for treatment, requiring a comprehensive approach for prevention and treatment. Thus, this study sought to evaluate the efficacy of various bone allografts in the absorption and release of antibiotics. A specially designed high-absorbency, high-surface-area carrier graft composed of human demineralized cortical fibers and granulated cancellous bone (fibrous graft) was compared to different human bone allograft types. The groups tested here were three fibrous grafts with rehydration rates of 2.7, 4, and 8 mL/g (F(2.7), F(4), and F(8)); demineralized bone matrix (DBM); cortical granules; mineralized cancellous bone; and demineralized cancellous bone. The absorption capacity of the bone grafts was assessed after rehydration, the duration of absorption varied from 5 to 30 min, and the elution kinetics of gentamicin were determined over 21 days. Furthermore, antimicrobial activity was assessed using a zone of inhibition (ZOI) test with S. aureus. The fibrous grafts exhibited the greatest tissue matrix absorption capacity, while the mineralized cancellous bone revealed the lowest matrix-bound absorption capacity. For F(2.7) and F(4), a greater elution of gentamicin was observed from 4 h and continuously over the first 3 days when compared to the other grafts. Release kinetics were only marginally affected by the varied incubation times. The enhanced absorption capacity of the fibrous grafts resulted in a prolonged antibiotic release and activity. Therefore, fibrous grafts can serve as suitable carrier grafts, as they are able to retain fluids such as antibiotics at their intended destinations, are easy to handle, and allow for a prolonged antibiotic release. Application of these fibrous grafts can enable surgeons to provide longer courses of antibiotic administration for septic orthopedic indications, thus minimizing infections.
Keywords: allograft; antibiotics; bone; grafting material; infection.
Conflict of interest statement
The authors A.H., V.E., N.A., and J.B. are employees of the German Institute for Cell and Tissue Replacement (DIZG, gemeinnützige GmbH), a nonprofit provider of sterile allografts. The other authors declare no conflict of interest.
Figures
Similar articles
-
Osteogenic protein-1 for long bone nonunion: an evidence-based analysis.Ont Health Technol Assess Ser. 2005;5(6):1-57. Epub 2005 Apr 1. Ont Health Technol Assess Ser. 2005. PMID: 23074475 Free PMC article.
-
Which type of bone releases the most vancomycin? Comparison of spongious bone, cortical powder and cortico-spongious bone.Cell Tissue Bank. 2020 Mar;21(1):131-137. doi: 10.1007/s10561-019-09806-2. Epub 2019 Dec 21. Cell Tissue Bank. 2020. PMID: 31865504
-
Incorporation of impacted morselized bone allografts in rabbits.Transplant Proc. 2005 Jul-Aug;37(6):2802-4. doi: 10.1016/j.transproceed.2005.05.043. Transplant Proc. 2005. PMID: 16182814
-
Allograft Bone as Antibiotic Carrier.J Bone Jt Infect. 2017 Jan 1;2(1):52-62. doi: 10.7150/jbji.17466. eCollection 2017. J Bone Jt Infect. 2017. PMID: 28529864 Free PMC article. Review.
-
Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery.J Orthop Trauma. 2019 Apr;33(4):203-213. doi: 10.1097/BOT.0000000000001420. J Orthop Trauma. 2019. PMID: 30633080 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

