Bactericidal Activity of Silver Nanoparticles on Oral Biofilms Related to Patients with and without Periodontal Disease
- PMID: 37367275
- PMCID: PMC10299358
- DOI: 10.3390/jfb14060311
Bactericidal Activity of Silver Nanoparticles on Oral Biofilms Related to Patients with and without Periodontal Disease
Abstract
Background and objectives: Periodontal disease (PD) is a multifactorial oral disease regularly caused by bacterial biofilms. Silver nanoparticles (AgNP) have offered good antimicrobial activity; moreover, there is no available scientific information related to their antimicrobial effects in biofilms from patients with PD. This study reports the bactericidal activity of AgNP against oral biofilms related to PD.
Materials and methods: AgNP of two average particle sizes were prepared and characterized. Sixty biofilms were collected from patients with (30 subjects) and without PD (30 subjects). Minimal inhibitory concentrations of AgNP were calculated and the distribution of bacterial species was defined by polymerase chain reaction.
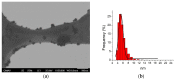
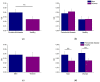
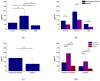
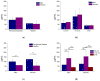
Results: Well-dispersed sizes of AgNP were obtained (5.4 ± 1.3 and 17.5 ± 3.4 nm) with an adequate electrical stability (-38.2 ± 5.8 and -32.6 ± 5.4 mV, respectively). AgNP showed antimicrobial activities for all oral samples; however, the smaller AgNP had significantly the most increased bactericidal effects (71.7 ± 39.1 µg/mL). The most resistant bacteria were found in biofilms from PD subjects (p < 0.05). P. gingivalis, T. denticola, and T. forsythia were present in all PD biofilms (100%).
Conclusions: The AgNP showed efficient bactericidal properties as an alternative therapy for the control or progression of PD.
Keywords: anti-bacterial agents; biofilms; humans; metal nanoparticles; periodontal diseases; silver.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Antibacterial Effect of Silver Nanoparticles against Oral Biofilms in Subjects with Motor and Intellectual Disabilities.J Funct Biomater. 2024 Jul 10;15(7):191. doi: 10.3390/jfb15070191. J Funct Biomater. 2024. PMID: 39057312 Free PMC article.
-
Site-specific development of periodontal disease is associated with increased levels of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia in subgingival plaque.J Periodontol. 2008 Apr;79(4):670-6. doi: 10.1902/jop.2008.070398. J Periodontol. 2008. PMID: 18380560
-
Formulation Optimization of Chitosan-Stabilized Silver Nanoparticles Using In Vitro Antimicrobial Assay.J Pharm Sci. 2019 Feb;108(2):1007-1016. doi: 10.1016/j.xphs.2018.09.011. Epub 2018 Sep 19. J Pharm Sci. 2019. PMID: 30244012
-
Antimicrobial Activity of a Colloidal AgNP Suspension Demonstrated In Vitro against Monoculture Biofilms: Toward a Novel Tooth Disinfectant for Treating Dental Caries.Adv Dent Res. 2018 Feb;29(1):117-123. doi: 10.1177/0022034517736495. Adv Dent Res. 2018. PMID: 29355416
-
Silver nanoparticles in dentistry.Dent Mater. 2017 Oct;33(10):1110-1126. doi: 10.1016/j.dental.2017.07.002. Epub 2017 Aug 2. Dent Mater. 2017. PMID: 28779891 Review.
Cited by
-
Microbiological evaluation of vitamin C rich acerola mediated silver and copperoxide nanogel in treatment of periodontitis with and without diabetes mellitus.J Oral Biol Craniofac Res. 2024 Nov-Dec;14(6):682-691. doi: 10.1016/j.jobcr.2024.09.011. Epub 2024 Sep 25. J Oral Biol Craniofac Res. 2024. PMID: 39381541 Free PMC article.
-
Current applications and future perspectives on rare-earth-based materials in stomatology.iScience. 2025 Jul 26;28(9):113220. doi: 10.1016/j.isci.2025.113220. eCollection 2025 Sep 19. iScience. 2025. PMID: 40822902 Free PMC article. Review.
-
Biosynthesis and Characterization of Silver Nanoparticles and Simvastatin Association in Titanium Biofilms.Pharmaceuticals (Basel). 2024 Nov 29;17(12):1612. doi: 10.3390/ph17121612. Pharmaceuticals (Basel). 2024. PMID: 39770455 Free PMC article.
-
Antibacterial and Anti-Adherence Efficacy of Silver Nanoparticles Against Endodontic Biofilms: An In Vitro and Ex Vivo Study.Pharmaceutics. 2025 Jun 26;17(7):831. doi: 10.3390/pharmaceutics17070831. Pharmaceutics. 2025. PMID: 40733040 Free PMC article.
-
Innovative strategies targeting oral microbial dysbiosis: unraveling mechanisms and advancing therapies for periodontitis.Front Cell Infect Microbiol. 2025 Apr 30;15:1556688. doi: 10.3389/fcimb.2025.1556688. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40370404 Free PMC article. Review.
References
-
- Chapple I.L.C., Mealey B.L., Van Dyke T.E., Bartold P.M., Dommisch H., Eickholz P., Geisinger M.L., Genco R.J., Glogauer M., Goldstein M., et al. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018;89((Suppl. S1)):S74–S84. doi: 10.1002/JPER.17-0719. - DOI - PubMed
-
- Gasner N., Schure R. Periodontal Disease—StatPearls—NCBI Bookshelf. [(accessed on 19 May 2023)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK554590/
Grants and funding
LinkOut - more resources
Full Text Sources

