Osteoblastic and Bacterial Response of Hybrid Dental Implants
- PMID: 37367285
- PMCID: PMC10299178
- DOI: 10.3390/jfb14060321
Osteoblastic and Bacterial Response of Hybrid Dental Implants
Abstract
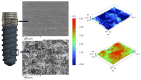
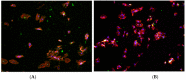
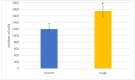
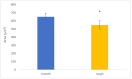
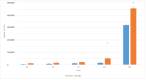
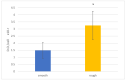
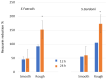
Bacterial infections in dental implants generate peri-implantitis disease that causes bone loss and the mobility of the dental implant. It is well known that specific ranges of roughness favor the proliferation of bacteria, and it is for this reason that new dental implants called hybrids have appeared. These implants have a smooth area in the coronal part and a rough surface in the apical part. The objective of this research is the physico-chemical characterization of the surface and the osteoblastic and microbiological behavior. One-hundred and eighty discs of titanium grade 3 with three different surfaces (smooth, smooth-rough, and completely rough) were studied. The roughness was determined by white light interferometry, and the wettability and surface energy by the sessile drop technique and the application of Owens and Wendt equations. Human osteoblast SaOS-2 was cultured to determine cell adhesion, proliferation, and differentiation. Microbiological studies were performed with two common bacterial strains in oral infection, E. faecalis and S. gordonii, at different times of culture. The roughness obtained for the smooth surface was Sa = 0.23 and for the rough surface it was 1.98 μm. The contact angles were more hydrophilic for the smooth surface (61.2°) than for the rough surface (76.1°). However, the surface energy was lower for the rough surface (22.70 mJ/m2) in both its dispersive and polar components than the smooth surface (41.77 mJ/m2). Cellular activity in adhesion, proliferation, and differentiation was much higher on rough surfaces than on smooth surfaces. After 6 h of incubation, the osteoblast number in rough surfaces was more than 32% higher in relation to the smooth surface. The cell area in smooth surfaces was higher than rough surfaces. The proliferation increased and the alkaline phosphatase presented a maximum after 14 days, with the mineral content of the cells being higher in rough surfaces. In addition, the rough surfaces showed greater bacterial proliferation at the times studied and in the two strains used. Hybrid implants sacrifice the good osteoblast behavior of the coronal part of the implant in order to obstruct bacterial adhesion. The following fact should be considered by clinicians: there is a possible loss of bone fixation when preventing peri-implantitis.
Keywords: bacteria; osteoblasts; peri-implantitis; roughness; titanium; wettability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ekelund J.A., Lindquist L.W., Carlsson G.E., Jemt T. Implant treatment in the edentulous mandible: A prospective study on Branemark system implants over more than 20 years. Int. J. Prosthodont. 2003;16:602–608. - PubMed
-
- Pjetursson B.E., Thoma D., Jung R., Zwahlen M., Zembic A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2012;23((Suppl. S6)):22–38. doi: 10.1111/j.1600-0501.2012.02546.x. - DOI - PubMed
-
- Branemark P.I., Adell R., Breine U., Hansson O., Lindström J., Ohlsson A. Intraosseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969;3:81–100. - PubMed
-
- Annunziata M., Guida L. The Effect of Titanium Surface Modifications on Dental Implant Osseointegration. Front. Oral Biol. Basel Kanger. 2015;17:62–77. - PubMed
LinkOut - more resources
Full Text Sources

