PEGylated Paclitaxel Nanomedicine Meets 3D Confinement: Cytotoxicity and Cell Behaviors
- PMID: 37367286
- PMCID: PMC10298934
- DOI: 10.3390/jfb14060322
PEGylated Paclitaxel Nanomedicine Meets 3D Confinement: Cytotoxicity and Cell Behaviors
Abstract
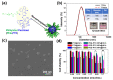
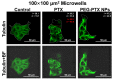
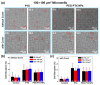
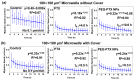
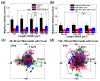
Investigating the effect of nanomedicines on cancer cell behavior in three-dimensional (3D) platforms is beneficial for evaluating and developing novel antitumor nanomedicines in vitro. While the cytotoxicity of nanomedicines on cancer cells has been widely studied on two-dimensional flat surfaces, there is little work using 3D confinement to assess their effects. This study aims to address this gap by applying PEGylated paclitaxel nanoparticles (PEG-PTX NPs) for the first time to treat nasopharyngeal carcinoma (NPC43) cells in 3D confinement consisting of microwells with different sizes and a glass cover. The cytotoxicity of the small molecule drug paclitaxel (PTX) and PEG-PTX NPs was studied in microwells with sizes of 50 × 50, 100 × 100, and 150 × 150 μm2 both with and without a concealed top cover. The impact of microwell confinement with varying sizes and concealment on the cytotoxicity of PTX and PEG-PTX NPs was analyzed by assessing NPC43 cell viability, migration speed, and cell morphology following treatment. Overall, microwell isolation was found to suppress drug cytotoxicity, and differences were observed in the time-dependent effects of PTX and PEG-PTX NPs on NPC43 cells in isolated and concealed microenvironments. These results not only demonstrate the effect of 3D confinement on nanomedicine cytotoxicity and cell behaviors but also provide a novel method to screen anticancer drugs and evaluate cell behaviors in vitro.
Keywords: 3D confinement; cell migration; microwell; nanomedicine; paclitaxel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

