Abscisic Acid: A Potential Secreted Effector Synthesized by Phytophagous Insects for Host-Plant Manipulation
- PMID: 37367305
- PMCID: PMC10299484
- DOI: 10.3390/insects14060489
Abscisic Acid: A Potential Secreted Effector Synthesized by Phytophagous Insects for Host-Plant Manipulation
Abstract
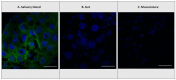
Abscisic acid (ABA) is an isoprenoid-derived plant signaling molecule involved in a wide variety of plant processes, including facets of growth and development as well as responses to abiotic and biotic stress. ABA had previously been reported in a wide variety of animals, including insects and humans. We used high-performance liquid chromatography-electrospray ionization tandem mass spectrometry (HPLC-(ESI)-MS/MS) to examine concentrations of ABA in 17 species of phytophagous insects, including gall- and non-gall-inducing species from all insect orders with species known to induce plant galls: Thysanoptera, Hemiptera, Lepidoptera, Coleoptera, Diptera, and Hymenoptera. We found ABA in insect species in all six orders, in both gall-inducing and non-gall-inducing species, with no tendency for gall-inducing insects to have higher concentrations. The concentrations of ABA in insects often markedly exceeded those typically found in plants, suggesting it is highly improbable that insects obtain all their ABA from their host plant via consumption and sequestration. As a follow-up, we used immunohistochemistry to determine that ABA localizes to the salivary glands in the larvae of the gall-inducing Eurosta solidaginis (Diptera: Tephritidae). The high concentrations of ABA, combined with its localization to salivary glands, suggest that insects are synthesizing and secreting ABA to manipulate their host plants. The pervasiveness of ABA among both gall- and non-gall-inducing insects and our current knowledge of the role of ABA in plant processes suggest that insects are using ABA to manipulate source-sink mechanisms of nutrient allocation or to suppress host-plant defenses. ABA joins the triumvirate of phytohormones, along with cytokinins (CKs) and indole-3-acetic acid (IAA), that are abundant, widespread, and localized to glandular organs in insects and used to manipulate host plants.
Keywords: abscisic acid (ABA); gall-inducing; manipulation of host plants; mobilizing sinks; non-gall-inducing; phytophagous; suppression of host defenses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Vishwakarma K., Upadhyay N., Kumar N., Yadav G., Singh J., Mishra R.K., Kumar V., Verma R., Upadhyay R.G., Pandey M., et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017;8:161. doi: 10.3389/fpls.2017.00161. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

