Balloon Dilatation in the Management of Congenital Obstructive Lesions of the Heart: Review of Author's Experiences and Observations-Part I
- PMID: 37367392
- PMCID: PMC10299120
- DOI: 10.3390/jcdd10060227
Balloon Dilatation in the Management of Congenital Obstructive Lesions of the Heart: Review of Author's Experiences and Observations-Part I
Abstract
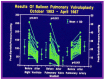
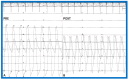
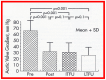
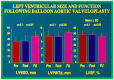
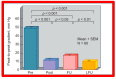
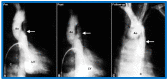
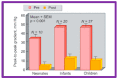
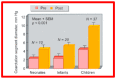
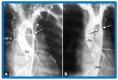
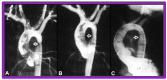
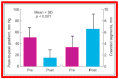
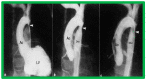
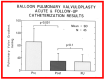
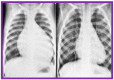
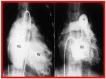
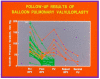
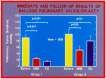
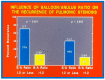
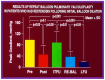
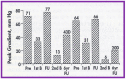
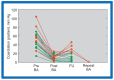
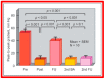
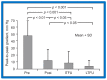
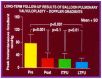
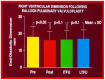
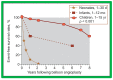
Balloon dilatation techniques became available to treat congenital obstructive lesions of the heart in the early/mid-1980s. The purpose of this review is to present the author's experiences and observations on the techniques and outcomes of balloon dilatation of pulmonary stenosis (PS), aortic stenosis (AS) and aortic coarctation (AC), both native and postsurgical re-coarctations. Balloon dilatation resulted in a reduction of peak pressure gradient across the obstructive lesion at the time of the procedure as well as at short-term and long-term follow-ups. Complications such as recurrence of stenosis, valvar insufficiency (for PS and AS cases) and aneurysm formation (for AC cases) have been reported, but infrequently. It was recommended that strategies be developed to prevent the reported complications.
Keywords: aortic aneurysm; aortic coarctation; aortic insufficiency; aortic stenosis; balloon angioplasty; balloon valvuloplasy; long-term results; pulmonary insufficiency; pulmonary stenosis.
Conflict of interest statement
The authors declare no conflict of interest.
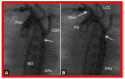
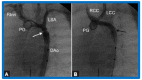
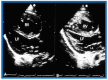
Figures

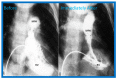

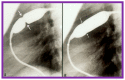
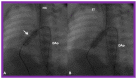

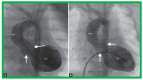


































References
-
- Grüntzig A., Schneider H.J. The percutaneous dilatation of chronic coronary stenoses--experiments and morphology. Schweiz. Med. Wochenschr. 1977;107:1588. (In German) - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

