Recasting Current Knowledge of Human Fetal Circulation: The Importance of Computational Models
- PMID: 37367405
- PMCID: PMC10299027
- DOI: 10.3390/jcdd10060240
Recasting Current Knowledge of Human Fetal Circulation: The Importance of Computational Models
Abstract
Computational hemodynamic simulations are becoming increasingly important for cardiovascular research and clinical practice, yet incorporating numerical simulations of human fetal circulation is relatively underutilized and underdeveloped. The fetus possesses unique vascular shunts to appropriately distribute oxygen and nutrients acquired from the placenta, adding complexity and adaptability to blood flow patterns within the fetal vascular network. Perturbations to fetal circulation compromise fetal growth and trigger the abnormal cardiovascular remodeling that underlies congenital heart defects. Computational modeling can be used to elucidate complex blood flow patterns in the fetal circulatory system for normal versus abnormal development. We present an overview of fetal cardiovascular physiology and its evolution from being investigated with invasive experiments and primitive imaging techniques to advanced imaging (4D MRI and ultrasound) and computational modeling. We introduce the theoretical backgrounds of both lumped-parameter networks and three-dimensional computational fluid dynamic simulations of the cardiovascular system. We subsequently summarize existing modeling studies of human fetal circulation along with their limitations and challenges. Finally, we highlight opportunities for improved fetal circulation models.
Keywords: cardiovascular lumped-parameter networks; computational fluid dynamics; congenital heart defects; fetal circulation; growth restriction; hemodynamics; patient-specific modeling; pediatric cardiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

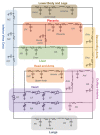
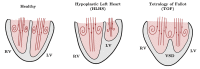
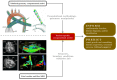
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

