Osteoderm Development during the Regeneration Process in Eurylepis taeniolata Blyth, 1854 (Scincidae, Sauria, Squamata)
- PMID: 37367476
- PMCID: PMC10299357
- DOI: 10.3390/jdb11020022
Osteoderm Development during the Regeneration Process in Eurylepis taeniolata Blyth, 1854 (Scincidae, Sauria, Squamata)
Abstract
Osteoderms are bony structures that develop within the dermal layer of the skin in vertebrates and are very often found in different lizard families. Lizard osteoderms are diverse in topography, morphology, and microstructure. Of particular interest are the compound osteoderms of skinks, which are a complex of several bone elements known as osteodermites. We present new data on the development and regeneration of compound osteoderms based on the results of a histological and Computed Microtomography (micro-CT) study of a scincid lizard: Eurylepis taeniolata. The specimens studied are stored in the herpetological collections of the Saint-Petersburg State University and Zoological Institute of the Russian Academy of Sciences located in St. Petersburg, Russia. The topography of osteoderms in the integuments of the original tail area and its regenerated part was studied. A comparative histological description of the original and regenerated osteoderms of Eurylepis taeniolata is presented for the first time. The first description of the development of compound osteoderm microstructure in the process of caudal regeneration is also presented.
Keywords: Eurylepis taeniolata; anatomy; development; histology; osteoderms; regeneration.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




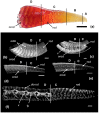

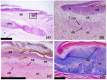

References
-
- Paluh D.J., Griffing A.H., Bauer A.M. Sheddable Armour: Identification of Osteoderms in the Integument of Geckolepis maculata (Gekkota) Afr. J. Herpetol. 2017;66:12–24. doi: 10.1080/21564574.2017.1281172. - DOI
-
- Vickaryous M., Williams C., Willan G., Kirby A., Herrel A., Kever L., Moazen M., Marghoub A., Rai S., Abzhanov A., et al. Histological Diversity and Evolution of Lizard Osteoderms. FASEB J. 2022;36 doi: 10.1096/fasebj.2022.36.S1.R3199. - DOI
-
- Bochaton C., De Buffrenil V., Lemoine M., Bailon S., Ineich I. Body Location and Tail Regeneration Effects on Osteoderms Morphology—Are They Useful Tools for Systematic, Paleontology, and Skeletochronology in Diploglossine Lizards (Squamata, Anguidae)? J. Morphol. 2015;276:1333–1344. doi: 10.1002/jmor.20421. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

