Jak2 and Jaw Muscles Are Required for Buccopharyngeal Membrane Perforation during Mouth Development
- PMID: 37367478
- PMCID: PMC10298892
- DOI: 10.3390/jdb11020024
Jak2 and Jaw Muscles Are Required for Buccopharyngeal Membrane Perforation during Mouth Development
Abstract
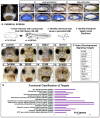
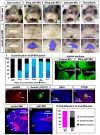
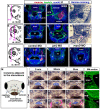
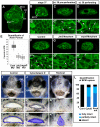
The mouth is a central feature of our face, without which we could not eat, breathe, or communicate. A critical and early event in mouth formation is the creation of a "hole" which connects the digestive system and the external environment. This hole, which has also been called the primary or embryonic mouth in vertebrates, is initially covered by a 1-2 cell layer thick structure called the buccopharyngeal membrane. When the buccopharyngeal membrane does not rupture, it impairs early mouth functions and may also lead to further craniofacial malformations. Using a chemical screen in an animal model (Xenopus laevis) and genetic data from humans, we determined that Janus kinase 2 (Jak2) has a role in buccopharyngeal membrane rupture. We have determined that decreased Jak2 function, using antisense morpholinos or a pharmacological antagonist, caused a persistent buccopharyngeal membrane as well as the loss of jaw muscles. Surprisingly, we observed that the jaw muscle compartments were connected to the oral epithelium that is continuous with the buccopharyngeal membrane. Severing such connections resulted in buccopharyngeal membrane buckling and persistence. We also noted puncta accumulation of F-actin, an indicator of tension, in the buccopharyngeal membrane during perforation. Taken together, the data has led us to a hypothesis that muscles are required to exert tension across the buccopharyngeal membrane, and such tension is necessary for its perforation.
Keywords: Xenopus laevis; buccopharyngeal membrane; choanal atresia; mouth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

