Foot-and-Mouth Disease Virus Induces Porcine Gasdermin E-Mediated Pyroptosis through the Protease Activity of 3Cpro
- PMID: 37367489
- PMCID: PMC10373541
- DOI: 10.1128/jvi.00686-23
Foot-and-Mouth Disease Virus Induces Porcine Gasdermin E-Mediated Pyroptosis through the Protease Activity of 3Cpro
Abstract
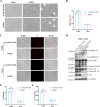
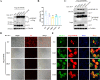
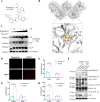
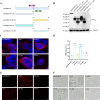
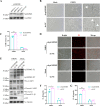
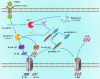
Foot-and-mouth disease (FMD) is an acute, highly contagious disease of cloven-hoofed animals caused by FMD virus (FMDV). Currently, the molecular pathogenesis of FMDV infection remains poorly understood. Here, we demonstrated that FMDV infection induced gasdermin E (GSDME)-mediated pyroptosis independent of caspase-3 activity. Further studies showed that FMDV 3Cpro cleaved porcine GSDME (pGSDME) at the Q271-G272 junction adjacent to the cleavage site (D268-A269) of porcine caspase-3 (pCASP3). The inhibition of enzyme activity of 3Cpro failed to cleave pGSDME and induce pyroptosis. Furthermore, overexpression of pCASP3 or 3Cpro-mediated cleavage fragment pGSDME-NT was sufficient to induce pyroptosis. Moreover, the knockdown of GSDME attenuated the pyroptosis caused by FMDV infection. Our study reveals a novel mechanism of pyroptosis induced by FMDV infection and might provide new insights into the pathogenesis of FMDV and the design of antiviral drugs. IMPORTANCE Although FMDV is an important virulent infectious disease virus, few reports have addressed its relationship with pyroptosis or pyroptosis factors, and most studies focus on the immune escape mechanism of FMDV. GSDME (DFNA5) was initially identified as being associated with deafness disorders. Accumulating evidence indicates that GSDME is a key executioner for pyroptosis. Here, we first demonstrate that pGSDME is a novel cleavage substrate of FMDV 3Cpro and can induce pyroptosis. Thus, this study reveals a previously unrecognized novel mechanism of pyroptosis induced by FMDV infection and might provide new insights into the design of anti-FMDV therapies and the mechanisms of pyroptosis induced by other picornavirus infections.
Keywords: 3Cpro; cleavage; foot-and-mouth disease virus; porcine gasdermin E; protease activity; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
