Marine Puupehenone and Puupehedione: Synthesis and Future Perspectives
- PMID: 37367647
- PMCID: PMC10301801
- DOI: 10.3390/md21060322
Marine Puupehenone and Puupehedione: Synthesis and Future Perspectives
Abstract
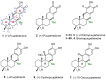
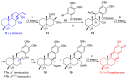
Puupehenone and puupehedione are natural products isolated from marine organisms. These compounds display a broad spectrum of biological activities, the in vitro antitubercular activity of puupehenone being a stand out, and are equipped with an interesting structural complexity. These products have served to stimulate the continual interest of the synthetic community. The first part of this article is a review of their total synthesis, using natural compounds which have the potential to be transformed into these marine compounds as starting materials; the synthetic routes employed to generate the basic skeleton; and the advances made to synthesize the pyran C ring with the required diastereoselectivity to obtain the natural products. Finally, this perspective shows a personal reflection of the authors on a possible unified and efficient retrosynthetic route that could allow easy access to these natural products, as well as their epimers at the C8 carbon and which could be used to address future biological issues in the production of pharmacologically active compounds.
Keywords: marine natural products; meroterpenoids; puupehedione; puupehenone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













Similar articles
-
Synthesis of Marine (−)-Pelorol and Future Perspectives.Mar Drugs. 2024 Sep 19;22(9):425. doi: 10.3390/md22090425. Mar Drugs. 2024. PMID: 39330306 Free PMC article. Review.
-
Enantiospecific Semisynthesis of Puupehedione-Type Marine Natural Products.J Org Chem. 2017 Dec 1;82(23):12914-12919. doi: 10.1021/acs.joc.7b02413. Epub 2017 Nov 7. J Org Chem. 2017. PMID: 29083172
-
First enantiospecific synthesis of the antitumor marine sponge metabolite (-)-15-oxopuupehenol from (-)-sclareol.Org Lett. 2005 Apr 14;7(8):1477-80. doi: 10.1021/ol047332j. Org Lett. 2005. PMID: 15816731
-
Selective Killing of Dormant Mycobacterium tuberculosis by Marine Natural Products.Antimicrob Agents Chemother. 2017 Jul 25;61(8):e00743-17. doi: 10.1128/AAC.00743-17. Print 2017 Aug. Antimicrob Agents Chemother. 2017. PMID: 28607021 Free PMC article.
-
Divergent Strategy in Marine Tetracyclic Meroterpenoids Synthesis.Mar Drugs. 2021 May 13;19(5):273. doi: 10.3390/md19050273. Mar Drugs. 2021. PMID: 34068313 Free PMC article. Review.
Cited by
-
Synthesis of Marine (−)-Pelorol and Future Perspectives.Mar Drugs. 2024 Sep 19;22(9):425. doi: 10.3390/md22090425. Mar Drugs. 2024. PMID: 39330306 Free PMC article. Review.
-
Unveiling Drimenol: A Phytochemical with Multifaceted Bioactivities.Plants (Basel). 2024 Sep 5;13(17):2492. doi: 10.3390/plants13172492. Plants (Basel). 2024. PMID: 39273976 Free PMC article. Review.
References
-
- Marcos I.S., Conde A., Moro R.F., Basabe P., Diez D., Urones J.G. Quinone/Hydroquinone Sesquiterpenes. Mini-Rev. Org. Chem. 2010;7:230. doi: 10.2174/157019310791384128. - DOI
-
- Djura P., Stierle D.B., Sullivan B., Faulkner D.J., Arnold E., Clardy J. Some metabolites of the marine sponges Smenospongia aurea and Smenospongia (polyfibrospongia) echina. J. Org. Chem. 1980;45:1435–1441. doi: 10.1021/jo01296a019. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- Projects 2020/00001014 and 2021/00000422/University of Seville
- Project Politec-Biomat: Red de Biomateriales en la Universidad de Sevilla/University of Sevilla
- projects UALFEDER 2020-FQM-B1989, PY20_01027/University of Almería
- CEIA3 PYC20 RE 060 UAL/Regional Government of Andalusia
- Horizon 2020-Research and Innovation Framework Program of the European Commission for the project 101022507 LAURELIN./European Union
LinkOut - more resources
Full Text Sources

