Biochemical Properties of a Cold-Active Chitinase from Marine Trichoderma gamsii R1 and Its Application to Preparation of Chitin Oligosaccharides
- PMID: 37367657
- PMCID: PMC10302006
- DOI: 10.3390/md21060332
Biochemical Properties of a Cold-Active Chitinase from Marine Trichoderma gamsii R1 and Its Application to Preparation of Chitin Oligosaccharides
Abstract
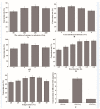
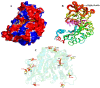
The enzymatic degradation of different chitin polymers into chitin oligosaccharides (COSs) is of great significance given their better solubility and various biological applications. Chitinase plays a pivotal role in the enzymatic preparation of COSs. Herein, a cold-adapted and efficient chitinase (ChiTg) from the marine Trichoderma gamsii R1 was purified and characterized. The optimal temperature of ChiTg was 40 °C, and the relative activity at 5 °C was above 40.1%. Meanwhile, ChiTg was active and stable from pH 4.0 to 7.0. As an endo-type chitinase, ChiTg exhibited the highest activity with colloidal chitin, then with ball-milled and powdery chitin. In addition, ChiTg showed high efficiency when hydrolyzing colloidal chitin at different temperatures, and the end products were mainly composed of COSs with one to three degrees of polymerization. Furthermore, the results of bioinformatics analysis revealed that ChiTg belongs to the GH18 family, and its acidic surface and the flexible structure of its catalytic site may contribute to its high activity in cold conditions. The results of this study provide a cold-active and efficient chitinase and ideas for its application regarding the preparation of COSs from colloidal chitin.
Keywords: Trichoderma gamsii; chitin oligosaccharides; chitinase; cold-adapted.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Amiri H., Aghbashlo M., Sharma M., Gaffey J., Manning L., Moosavi Basri S.M., Kennedy J.F., Gupta V.K., Tabatabaei M. Chitin and chitosan derived from crustacean waste valorization streams can support food systems and the UN Sustainable Development Goals. Nat. Food. 2022;3:822–828. doi: 10.1038/s43016-022-00591-y. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

