Laurequinone, a Lead Compound against Leishmania
- PMID: 37367658
- PMCID: PMC10304164
- DOI: 10.3390/md21060333
Laurequinone, a Lead Compound against Leishmania
Abstract
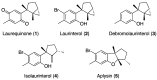
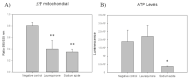
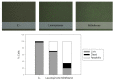
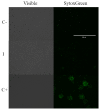
Among neglected tropical diseases, leishmaniasis is one of the leading causes, not only of deaths but also of disability-adjusted life years. This disease, caused by protozoan parasites of the genus Leishmania, triggers different clinical manifestations, with cutaneous, mucocutaneous, and visceral forms. As existing treatments for this parasitosis are not sufficiently effective or safe for the patient, in this work, different sesquiterpenes isolated from the red alga Laurencia johnstonii have been studied for this purpose. The different compounds were tested in vitro against the promastigote and amastigote forms of Leishmania amazonensis. Different assays were also performed, including the measurement of mitochondrial potential, determination of ROS accumulation, and chromatin condensation, among others, focused on the detection of the cell death process known in this type of organism as apoptosis-like. Five compounds were identified that displayed leishmanicidal activity: laurequinone, laurinterol, debromolaurinterol, isolaurinterol, and aplysin, showing IC50 values against promastigotes of 1.87, 34.45, 12.48, 10.09, and 54.13 µM, respectively. Laurequinone was the most potent compound tested and was shown to be more effective than the reference drug miltefosine against promastigotes. Different death mechanism studies carried out showed that laurequinone appears to induce programmed cell death or apoptosis in the parasite studied. The obtained results underline the potential of this sesquiterpene as a novel anti-kinetoplastid therapeutic agent.
Keywords: Laurencia; Leishmania amazonensis; laurequinone; leishmaniasis; sesquiterpene.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- World Health Organization Neglected Tropical Diseases (NTDs) 2021. [(accessed on 3 April 2023)]. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1.
-
- Pan American Health Organization—World Health Organization Leishmaniasis—Fact Sheet for Health Workers. 2017. [(accessed on 3 April 2023)]. Available online: https://www.paho.org/en.
-
- Marinho F.D.E.A., Gonçalves K.C., Oliveira S.S., Oliveira A.C., Bellio M., d’Avila-Levy C.M., Santos A.L., Branquinha M.H. Miltefosine induces programmed cell death in Leishmania amazonensis promastigotes. Mem. Inst. Oswaldo Cruz. 2011;106:507–509. doi: 10.1590/S0074-02762011000400021. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

