The Loss of Structural Integrity of 3D Chitin Scaffolds from Aplysina aerophoba Marine Demosponge after Treatment with LiOH
- PMID: 37367659
- PMCID: PMC10305591
- DOI: 10.3390/md21060334
The Loss of Structural Integrity of 3D Chitin Scaffolds from Aplysina aerophoba Marine Demosponge after Treatment with LiOH
Abstract
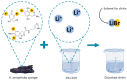
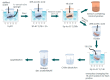
Aminopolysaccharide chitin is one of the main structural biopolymers in sponges that is responsible for the mechanical stability of their unique 3D-structured microfibrous and porous skeletons. Chitin in representatives of exclusively marine Verongiida demosponges exists in the form of biocomposite-based scaffolds chemically bounded with biominerals, lipids, proteins, and bromotyrosines. Treatment with alkalis remains one of the classical approaches to isolate pure chitin from the sponge skeleton. For the first time, we carried out extraction of multilayered, tube-like chitin from skeletons of cultivated Aplysina aerophoba demosponge using 1% LiOH solution at 65 °C following sonication. Surprisingly, this approach leads not only to the isolation of chitinous scaffolds but also to their dissolution and the formation of amorphous-like matter. Simultaneously, isofistularin-containing extracts have been obtained. Due to the absence of any changes between the chitin standard derived from arthropods and the sponge-derived chitin treated with LiOH under the same experimental conditions, we suggest that bromotyrosines in A. aerophoba sponge represent the target for lithium ion activity with respect to the formation of LiBr. This compound, however, is a well-recognized solubilizing reagent of diverse biopolymers including cellulose and chitosan. We propose a possible dissolution mechanism of this very special kind of sponge chitin.
Keywords: Aplysina aerophoba; bromotyrosines; chitin; dissolution; marine sponges.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures











Similar articles
-
On the Mechanical Properties of Microfibre-Based 3D Chitinous Scaffolds from Selected Verongiida Sponges.Mar Drugs. 2023 Aug 24;21(9):463. doi: 10.3390/md21090463. Mar Drugs. 2023. PMID: 37755076 Free PMC article.
-
Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge.Mar Drugs. 2019 Feb 22;17(2):131. doi: 10.3390/md17020131. Mar Drugs. 2019. PMID: 30813373 Free PMC article.
-
First Report on Chitin in a Non-Verongiid Marine Demosponge: The Mycale euplectellioides Case.Mar Drugs. 2018 Feb 20;16(2):68. doi: 10.3390/md16020068. Mar Drugs. 2018. PMID: 29461501 Free PMC article.
-
Sponge derived bromotyrosines: structural diversity through natural combinatorial chemistry.Nat Prod Commun. 2015 Jan;10(1):219-31. Nat Prod Commun. 2015. PMID: 25920247 Review.
-
Chitin and chitosan: functional biopolymers from marine crustaceans.Mar Biotechnol (NY). 2006 May-Jun;8(3):203-26. doi: 10.1007/s10126-005-0097-5. Epub 2006 Mar 17. Mar Biotechnol (NY). 2006. PMID: 16532368 Review.
Cited by
-
Current and Expected Trends for the Marine Chitin/Chitosan and Collagen Value Chains.Mar Drugs. 2023 Nov 23;21(12):605. doi: 10.3390/md21120605. Mar Drugs. 2023. PMID: 38132926 Free PMC article. Review.
-
On the Mechanical Properties of Microfibre-Based 3D Chitinous Scaffolds from Selected Verongiida Sponges.Mar Drugs. 2023 Aug 24;21(9):463. doi: 10.3390/md21090463. Mar Drugs. 2023. PMID: 37755076 Free PMC article.
-
Marine Collagen and Chitin: Promising Applications in Interdisciplinary Fields.Mar Drugs. 2024 Aug 23;22(9):379. doi: 10.3390/md22090379. Mar Drugs. 2024. PMID: 39330260 Free PMC article.
References
-
- Ehrlich H. Blue Biotechnology. Volume 1. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2018. Chitin of Poriferan Origin as a Unique Biological Material; pp. 821–854.
-
- Muzzarelli R., Boudrant J., Meyer D., Manno N., DeMarchis M., Paoletti M. Current Views on Fungal Chitin/Chitosan, Human Chitinases, Food Preservation, Glucans, Pectins and Inulin: A Tribute to Henri Braconnot, Precursor of Thecarbohydrate Polymers Science, on the Chitin Bicentennial. Carbohydr. Polym. 2012;87:995–1012. doi: 10.1016/j.carbpol.2011.09.063. - DOI
-
- Ehrlich H. Chitin and Collagen as Universal and Alternative Templates in Biomineralization. Int. Geol. Rev. 2010;52:661–699. doi: 10.1080/00206811003679521. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

