Pentaketides and 5- p-Hydroxyphenyl-2-pyridone Derivative from the Culture Extract of a Marine Sponge-Associated Fungus Hamigera avellanea KUFA0732
- PMID: 37367669
- PMCID: PMC10304554
- DOI: 10.3390/md21060344
Pentaketides and 5- p-Hydroxyphenyl-2-pyridone Derivative from the Culture Extract of a Marine Sponge-Associated Fungus Hamigera avellanea KUFA0732
Abstract
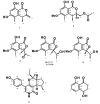
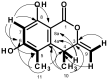
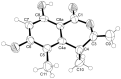
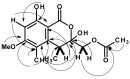
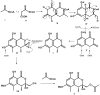
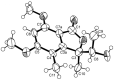
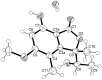
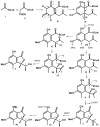
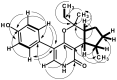
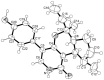
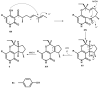
Five undescribed pentaketide derivatives, (R)-6,8-dihydroxy-4,5-dimethyl-3-methylidene-3,4-dihydro-1H-2-benzopyran-1-one (1), [(3S,4R)-3,8-dihydroxy-6-methoxy-4,5-dimethyl-1-oxo-3,4-dihydro-1H-isochromen-3-yl]methyl acetate (2), (R)-5, 7-dimethoxy-3-((S)-(1-hydroxyethyl)-3,4-dimethylisobenzofuran-1(3H)-one (4b), (S)-7-hydroxy-3-((S)-1-hydroxyethyl)-5-methoxy-3,4-dimethylisobenzofuran 1(3H)-one (5), and a p-hydroxyphenyl-2-pyridone derivative, avellaneanone (6), were isolated together with the previously reported (R)-3-acetyl-7-hydroxy-5-methoxy-3,4-dimethylisobenzofuran-1(3H)-one (3), (R)-7-hydroxy-3-((S)-1-hydroxyethyl)-5-methoxy-3,4-dimethylisobenzofuran-1(3H)-one (4a) and isosclerone (7), from the ethyl acetate extract of a culture of a marine sponge-derived fungus, Hamigera avellanea KUFA0732. The structures of the undescribed compounds were elucidated using 1D and 2D NMR, as well as high-resolution mass spectral analyses. The absolute configurations of the stereogenic carbons in 1, 4b, 5, and 6 were established by X-ray crystallographic analysis. The absolute configurations of C-3 and C-4 in 2 were determined by ROESY correlations and on the basis of their common biosynthetic origin with 1. The crude fungal extract and the isolated compounds 1, 3, 4b, 5, 6, and 7 were assayed for their growth inhibitory activity against various plant pathogenic fungi viz. Alternaria brassicicola, Bipolaris oryzae, Colletotrichum capsici, C. gloeosporiodes, Curvularia oryzae, Fusarium semitectum, Lasiodiplodia theobromae, Phytophthora palmivora, Pyricularia oryzae, Rhizoctonia oryzae and Sclerotium rolfsii.
Keywords: 5-p-hydroxy-2-pyridone; Aspergillaceae; Hamigera avellanea; anti-plant pathogenic fungal activity; dihydrochromone; marine sponge-associated fungus; pentaketides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Breinholt J., Kjaer A., Olsen C.E., Rassing B.R. A bis-formamidodiphenylbutadiene from the fungus Hamigera avellanea. Acta Chem. Scand. 1996;50:643–645. doi: 10.3891/acta.chem.scand.50-0643. - DOI
-
- Breinholt J., Kjaer A., Olsen C.E., Rassing B.R., Rosendahl C.N. Hamigerone and dihydrohamigerone: Two acetate-derived, antifungal metabolites from Hamigera avellanea. Acta Chem. Scand. 1997;51:1241–1244. doi: 10.3891/acta.chem.scand.51-1241. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

