Cyanobacteria: A Promising Source of Antifungal Metabolites
- PMID: 37367684
- PMCID: PMC10300848
- DOI: 10.3390/md21060359
Cyanobacteria: A Promising Source of Antifungal Metabolites
Abstract
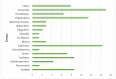
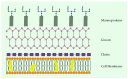
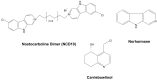
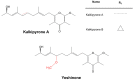
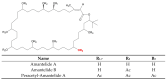
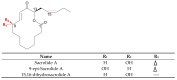
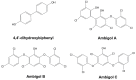
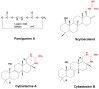
Cyanobacteria are a rich source of secondary metabolites, and they have received a great deal of attention due to their applicability in different industrial sectors. Some of these substances are known for their notorious ability to inhibit fungal growth. Such metabolites are very chemically and biologically diverse. They can belong to different chemical classes, including peptides, fatty acids, alkaloids, polyketides, and macrolides. Moreover, they can also target different cell components. Filamentous cyanobacteria have been the main source of these compounds. This review aims to identify the key features of these antifungal agents, as well as the sources from which they are obtained, their major targets, and the environmental factors involved when they are being produced. For the preparation of this work, a total of 642 documents dating from 1980 to 2022 were consulted, including patents, original research, review articles, and theses.
Keywords: action mechanism; alkaloids; antifungal agents; cyanobacteria; peptides; secondary metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



















References
-
- Kendrick B. Fungi: Ecological Importance and Impact on Humans. eLS. 2011:1–5. doi: 10.1002/9780470015902.a0000369.pub2. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

