Limited emergence of resistance to integrase strand transfer inhibitors (INSTIs) in ART-experienced participants failing dolutegravir-based antiretroviral therapy: a cross-sectional analysis of a Northeast Nigerian cohort
- PMID: 37367727
- PMCID: PMC10393879
- DOI: 10.1093/jac/dkad195
Limited emergence of resistance to integrase strand transfer inhibitors (INSTIs) in ART-experienced participants failing dolutegravir-based antiretroviral therapy: a cross-sectional analysis of a Northeast Nigerian cohort
Abstract
Background: Due to the high prevalence of resistance to NNRTI-based ART since 2018, consolidated recommendations from the WHO have indicated dolutegravir as the preferred drug of choice for HIV treatment globally. There is a paucity of resistance outcome data from HIV-1 non-B subtypes circulating across West Africa.
Aims: We characterized the mutational profiles of persons living with HIV from a cross-sectional cohort in North-East Nigeria failing a dolutegravir-based ART regimen.
Methods: WGS of plasma samples collected from 61 HIV-1-infected participants following virological failure of dolutegravir-based ART were sequenced using the Illumina platform. Sequencing was successfully completed for samples from 55 participants. Following quality control, 33 full genomes were analysed from participants with a median age of 40 years and median time on ART of 9 years. HIV-1 subtyping was performed using SNAPPy.
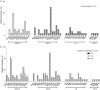
Results: Most participants had mutational profiles reflective of exposure to previous first- and second-line ART regimens comprised NRTIs and NNRTIs. More than half of participants had one or more drug resistance-associated mutations (DRMs) affecting susceptibility to NRTIs (17/33; 52%) and NNRTIs (24/33; 73%). Almost a quarter of participants (8/33; 24.4%) had one or more DRMs affecting tenofovir susceptibility. Only one participant, infected with HIV-1 subtype G, had evidence of DRMs affecting dolutegravir susceptibility-this was characterized by the T66A, G118R, E138K and R263K mutations.
Conclusions: This study found a low prevalence of resistance to dolutegravir; the data are therefore supportive of the continual rollout of dolutegravir as the primary first-line regimen for ART-naive participants and the preferred switch to second-line ART across the region. However, population-level, longer-term data collection on dolutegravir outcomes are required to further guide implementation and policy action across the region.
© The Author(s) 2023. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures

References
-
- Gupta RK, Jordan MR, Sultan BJ et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 2012; 380: 1250–8. 10.1016/S0140-6736(12)61038-1 - DOI - PMC - PubMed
-
- Gupta RK, Gregson J, Parkin N et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18: 346–55. 10.1016/S1473-3099(17)30702-8 - DOI - PMC - PubMed
-
- WHO . Update of recommendations on first- and second-line antiretroviral regimens. 2019. https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.1....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

