An Electrochemistry and Computational Study at an Electrified Liquid-Liquid Interface for Studying Beta-Amyloid Aggregation
- PMID: 37367788
- PMCID: PMC10303370
- DOI: 10.3390/membranes13060584
An Electrochemistry and Computational Study at an Electrified Liquid-Liquid Interface for Studying Beta-Amyloid Aggregation
Abstract
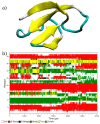
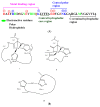
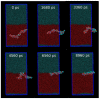
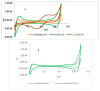
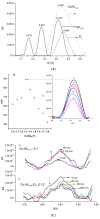
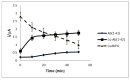
Amphiphilic peptides, such as Aß amyloids, can adsorb at an interface between two immiscible electrolyte solutions (ITIES). Based on previous work (vide infra), a hydrophilic/hydrophobic interface is used as a simple biomimetic system for studying drug interactions. The ITIES provides a 2D interface to study ion-transfer processes associated with aggregation, as a function of Galvani potential difference. Here, the aggregation/complexation behaviour of Aβ(1-42) is studied in the presence of Cu (II) ions, together with the effect of a multifunctional peptidomimetic inhibitor (P6). Cyclic and differential pulse voltammetry proved to be particularly sensitive to the detection of the complexation and aggregation of Aβ(1-42), enabling estimations of changes in lipophilicity upon binding to Cu (II) and P6. At a 1:1 ratio of Cu (II):Aβ(1-42), fresh samples showed a single DPV (Differential Pulse Voltammetry) peak half wave transfer potential (E1/2) at 0.40 V. Upon increasing the ratio of Cu (II) two-fold, fluctuations were observed in the DPVs, indicating aggregation. The approximate stoichiometry and binding properties of Aβ(1-42) during complexation with Cu (II) were determined by performing a differential pulse voltammetry (DPV) standard addition method, which showed two binding regimes. A pKa of 8.1 was estimated, with a Cu:Aβ1-42 ratio~1:1.7. Studies using molecular dynamics simulations of peptides at the ITIES show that Aβ(1-42) strands interact through the formation of β-sheet stabilised structures. In the absence of copper, binding/unbinding is dynamic, and interactions are relatively weak, leading to the observation of parallel and anti-parallel arrangements of β-sheet stabilised aggregates. In the presence of copper ions, strong binding occurs between a copper ion and histidine residues on two peptides. This provides a convenient geometry for inducing favourable interactions between folded β-sheet structures. Circular Dichroism spectroscopy (CD spectroscopy) was used to support the aggregation behaviour of the Aβ(1-42) peptides following the addition of Cu (II) and P6 to the aqueous phase.
Keywords: aggregation; beta-amyloid; copper binding; drug–peptide interactions; electrified liquid–liquid interface; molecular dynamic simulations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Aggregation pathways of the amyloid β(1-42) peptide depend on its colloidal stability and ordered β-sheet stacking.Langmuir. 2012 Sep 4;28(35):12711-21. doi: 10.1021/la3021436. Epub 2012 Aug 22. Langmuir. 2012. PMID: 22870885 Free PMC article.
-
The second Cu(II)-binding site in a proton-rich environment interferes with the aggregation of amyloid-beta(1-40) into amyloid fibrils.Biochemistry. 2009 Nov 17;48(45):10724-32. doi: 10.1021/bi9012935. Biochemistry. 2009. PMID: 19824649
-
Impact of Cu(II) Binding on Structures and Dynamics of Aβ42 Monomer and Dimer: Molecular Dynamics Study.ACS Chem Neurosci. 2016 Oct 19;7(10):1348-1363. doi: 10.1021/acschemneuro.6b00109. Epub 2016 Aug 16. ACS Chem Neurosci. 2016. PMID: 27454036
-
Electrochemistry of Alzheimer Disease Amyloid Beta Peptides.Curr Med Chem. 2018;25(33):4066-4083. doi: 10.2174/0929867325666180214112536. Curr Med Chem. 2018. PMID: 29446720 Review.
-
Molecular Dynamics Simulation Studies on the Aggregation of Amyloid-β Peptides and Their Disaggregation by Ultrasonic Wave and Infrared Laser Irradiation.Molecules. 2022 Apr 12;27(8):2483. doi: 10.3390/molecules27082483. Molecules. 2022. PMID: 35458686 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

