Synthesis and Oxygen Mobility of Bismuth Cerates and Titanates with Pyrochlore Structure
- PMID: 37367802
- PMCID: PMC10302842
- DOI: 10.3390/membranes13060598
Synthesis and Oxygen Mobility of Bismuth Cerates and Titanates with Pyrochlore Structure
Abstract
Synthesis and study of materials based on bismuth cerates and titanates were carried out. Complex oxides Bi1.6Y0.4Ti2O7 were synthesized by the citrate route; Bi2Ce2O7 and Bi1.6Y0.4Ce2O7-by the Pechini method. The structural characteristics of materials after conventional sintering at 500-1300 °C were studied. It is demonstrated that the formation of a pure pyrochlore phase, Bi1.6Y0.4Ti2O7, occurs after high-temperature calcination. Complex oxides Bi2Ce2O7 and Bi1.6Y0.4Ce2O7 have a pyrochlore structure formed at low temperatures. Yttrium doping of bismuth cerate lowers the formation temperature of the pyrochlore phase. As a result of calcination at high temperatures, the pyrochlore phase transforms into the CeO2-like fluorite phase enriched by bismuth oxide. The influence of radiation-thermal sintering (RTS) conditions using e-beams was studied as well. In this case, dense ceramics are formed even at sufficiently low temperatures and short processing times. The transport characteristics of the obtained materials were studied. It has been shown that bismuth cerates have high oxygen conductivity. Conclusions are drawn about the oxygen diffusion mechanism for these systems. The materials studied are promising for use as oxygen-conducting layers in composite membranes.
Keywords: bismuth cerate; bismuth titanate; oxygen mobility; oxygen separation membranes; pyrochlores.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

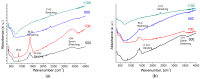

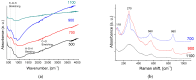



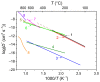
References
-
- Shlyakhtina A.V., Shcherbakova L.G. New solid electrolytes of the pyrochlore family. Russ. J. Electrochem. 2012;48:1–25. doi: 10.1134/S1023193512010144. - DOI
-
- Julbe A., Farrusseng D., Guizard C. Limitations and potentials of oxygen transport dense and porous ceramic membranes for oxidation reactions. Catal. Today. 2005;104:102–113. doi: 10.1016/j.cattod.2005.03.075. - DOI
-
- Zhang C., Huang Y., Zeng L., He Y., Yu P., Luo H. Effects of Bi Substitution on the cobalt-free 60wt.%Ce0.9Pr0.1O2−δ-40wt.%Pr0.6Sr0.4Fe1−xBixO3−δ oxygen transport membranes. Processes. 2021;9:1767. doi: 10.3390/pr9101767. - DOI
-
- Sunarso J., Motuzas J., Liuc S., Diniz da Costa J.C. Bi-doping effects on the structure and oxygen permeation properties of BaSc0.1Co0.9O3−δ perovskite membranes. J. Membr. Sci. 2010;361:120–125. doi: 10.1016/j.memsci.2010.06.001. - DOI
-
- Phair J.W., Badwal S.P.S. Materials for separation membranes in hydrogen and oxygen production and future power generation. Sci. Tech. Adv. Mater. 2006;7:792–805. doi: 10.1016/j.stam.2006.11.005. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

