Interaction of Quercetin, Cyanidin, and Their O-Glucosides with Planar Lipid Models: Implications for Their Biological Effects
- PMID: 37367804
- PMCID: PMC10300802
- DOI: 10.3390/membranes13060600
Interaction of Quercetin, Cyanidin, and Their O-Glucosides with Planar Lipid Models: Implications for Their Biological Effects
Abstract
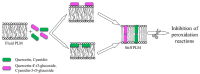
Flavonoids are specialized metabolites produced by plants, as free aglycones or as glycosylated derivatives, which are particularly endowed with a variety of beneficial health properties. The antioxidant, anti-inflammatory, antimicrobial, anticancer, antifungal, antiviral, anti-Alzheimer's, anti-obesity, antidiabetic, and antihypertensive effects of flavonoids are now known. These bioactive phytochemicals have been shown to act on different molecular targets in cells including the plasma membrane. Due to their polyhydroxylated structure, lipophilicity, and planar conformation, they can either bind at the bilayer interface or interact with the hydrophobic fatty acid tails of the membrane. The interaction of quercetin, cyanidin, and their O-glucosides with planar lipid membranes (PLMs) similar in composition to those of the intestine was monitored using an electrophysiological approach. The obtained results show that the tested flavonoids interact with PLM and form conductive units. The modality of interaction with the lipids of the bilayer and the alteration of the biophysical parameters of PLMs induced by the tested substances provided information on their location in the membrane, helping to elucidate the mechanism of action which underlies some pharmacological properties of flavonoids. To our knowledge, the interaction of quercetin, cyanidin, and their O-glucosides with PLM surrogates of the intestinal membrane has never been previously monitored.
Keywords: antioxidant; conductive unit; cyanidin; cyanidin-3-O-glucoside; interface active compounds; planar lipid membrane; polyphenols; quercetin; quercetin-4′-O-glucoside.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

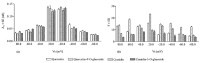
References
-
- Murota K., Mitsukuni Y., Ichikawa M., Tsushida T., Miyamoto S., Terao J. Quercetin-4′-glucoside is more potent than quercetin-3-glucoside in protection of rat intestinal mucosa homogenates against iron ion-induced lipid peroxidation. J. Agric. Food Chem. 2004;52:1907–1912. doi: 10.1021/jf035151a. - DOI - PubMed
LinkOut - more resources
Full Text Sources

