Sintering Aid Strategy for Promoting Oxygen Reduction Reaction on High-Performance Double-Layer LaNi0.6Fe0.4O3-δ Composite Electrode for Devices Based on Solid-State Membranes
- PMID: 37367807
- PMCID: PMC10302646
- DOI: 10.3390/membranes13060603
Sintering Aid Strategy for Promoting Oxygen Reduction Reaction on High-Performance Double-Layer LaNi0.6Fe0.4O3-δ Composite Electrode for Devices Based on Solid-State Membranes
Abstract
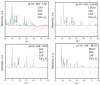
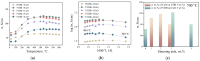
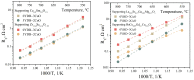
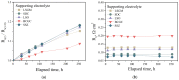
Strontium and cobalt-free LaNi0.6Fe0.4O3-δ is considered one of the most promising electrodes for solid-state electrochemical devices. LaNi0.6Fe0.4O3-δ has high electrical conductivity, a suitable thermal expansion coefficient, satisfactory tolerance to chromium poisoning, and chemical compatibility with zirconia-based electrolytes. The disadvantage of LaNi0.6Fe0.4O3-δ is its low oxygen-ion conductivity. In order to increase the oxygen-ion conductivity, a complex oxide based on a doped ceria is added to the LaNi0.6Fe0.4O3-δ. However, this leads to a decrease in the conductivity of the electrode. In this case, a two-layer electrode with a functional composite layer and a collector layer with the addition of sintering additives should be used. In this study, the effect of sintering additives (Bi0.75Y0.25O2-δ and CuO) in the collector layer on the performance of LaNi0.6Fe0.4O3-δ-based highly active electrodes in contact with the most common solid-state membranes (Zr0.84Sc0.16O2-δ, Ce0.8Sm0.2O2-δ, La0.85Sr0.15Ga0.85Mg0.15O3-δ, La10(SiO4)6O3-δ, and BaCe0.89Gd0.1Cu0.01O3-δ) was investigated. It was shown that LaNi0.6Fe0.4O3-δ has good chemical compatibility with the abovementioned membranes. The best electrochemical activity (polarization resistance about 0.02 Ohm cm2 at 800 °C) was obtained for the electrode with 5 wt.% Bi0.75Y0.25O1.5 and 2 wt.% CuO in the collector layer.
Keywords: DRT; LNF; LaNi0.6Fe0.4O3–δ; SOFC; complex oxide; distribution of relaxation times; electrode; membranes; polarization resistance; sintering aids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zhai H., Wu Z., Fang Z. Recent progress of Ga2O3-based gas sensors. Ceram. Int. 2022;48:24213–24233. doi: 10.1016/j.ceramint.2022.06.066. - DOI
-
- Lai H., II T.A.A. Life cycle analyses of SOFC/gas turbine hybrid power plants accounting for long-term degradation effects. J. Clean. Product. 2023;412:137411. doi: 10.1016/j.jclepro.2023.137411. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

