Nutritional Intervention as a Complementary Neuroprotective Approach against Propionic Acid-Induced Neurotoxicity and Associated Biochemical Autistic Features in Rat Pups
- PMID: 37367896
- PMCID: PMC10302727
- DOI: 10.3390/metabo13060738
Nutritional Intervention as a Complementary Neuroprotective Approach against Propionic Acid-Induced Neurotoxicity and Associated Biochemical Autistic Features in Rat Pups
Abstract
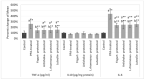
Since there is no known cure for autism spectrum disorder (ASD), its incidence rate is on the rise. Common comorbidities like gastrointestinal problems are observed as common signs of ASD and play a major role in controlling social and behavioral symptoms. Although there is a lot of interest in dietary treatments, no harmony exists with regard to the ideal nutritional therapy. To better direct prevention and intervention measures for ASD, the identification of risk and protective factors is required. Through the use of a rat model, our study aims to assess the possible danger of exposure to neurotoxic doses of propionic acid (PPA) and the nutritional protective effects of prebiotics and probiotics. Here, we conducted a biochemical assessment of the effects of dietary supplement therapy in the PPA model of autism. We used 36 male Sprague Dawley albino rat pups divided into six groups. Standard food and drink were given to the control group. The PPA-induced ASD model was the second group; it was fed a conventional diet for 27 days before receiving 250 mg/kg of PPA orally for three days. The four other groups were given 3 mL/kg of yoghurt daily, 400 mg/Kg of artichokes daily, 50 mg/kg of luteolin daily and Lacticaseibacillus rhamnosus GG at 0.2 mL daily for 27 days before being given PPA (250 mg/kg BW) for three days along with their normal diet. All groups had their brain homogenates tested for biochemical markers, which included gamma-aminobutyric acid (GABA), glutathione peroxidase 1 (GPX1), glutathione (GSH), interleukin 6 (IL-6), interleukin 10 (IL-10) and tumor necrosis factor-alpha (TNF). When compared with the control group, the PPA-induced model presented increased oxidative stress and neuroinflammation but groups treated with all four dietary therapies presented improvements in biochemical characteristics for oxidative stress and neuroinflammation. As all of the therapies show sufficient anti-inflammatory and antioxidant effects, they can be used as a useful dietary component to help prevent ASD.
Keywords: Lacticaseibacillus rhamnosus; anti-inflammatory; antioxidants; artichokes; autism spectrum disorder; luteolin; probiotics; propionic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Taniya M.A., Chung H.J., Al Mamun A., Alam S., Aziz M.A., Emon N.U., Islam M.M., Hong S.S., Podder B.R., Ara Mimi A., et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022;12:998. doi: 10.3389/fcimb.2022.915701. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

