Adavosertib (AZD1775) does not prolong the QTc interval in patients with advanced solid tumors: a phase I open-label study
- PMID: 37368100
- PMCID: PMC10326086
- DOI: 10.1007/s00280-023-04555-2
Adavosertib (AZD1775) does not prolong the QTc interval in patients with advanced solid tumors: a phase I open-label study
Abstract
Purpose: Adavosertib is a small-molecule, ATP-competitive inhibitor of Wee1 kinase. Molecularly targeted oncology agents have the potential to increase the risk of cardiovascular events, including prolongation of QT interval and associated cardiac arrhythmias. This study investigated the effect of adavosertib on the QTc interval in patients with advanced solid tumors.
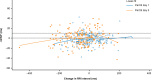
Methods: Eligible patients were ≥ 18 years of age with advanced solid tumors for which no standard therapy existed. Patients received adavosertib 225 mg twice daily on days 1-2 at 12-h intervals and once on day 3. Patients underwent digital 12-lead electrocardiogram and pharmacokinetic assessments pre-administration and time-matched assessments during the drug administration period. The relationship between maximum plasma drug concentration (Cmax) and baseline-adjusted corrected QT interval by Fridericia (QTcF) was estimated using a prespecified linear mixed-effects model.
Results: Twenty-one patients received adavosertib. Concentration-QT modeling of ΔQTcF and the upper limit of the 90% confidence interval corresponding to the geometric mean of Cmax observed on days 1 and 3 were below the threshold for regulatory concern (not > 10 ms). No significant relationship between ΔQTcF (vs baseline) and adavosertib concentration was identified (P = 0.27). Pharmacokinetics and the adverse event (AE) profile were consistent with previous studies at this dose. Eleven (52.4%) patients experienced 17 treatment-related AEs in total, including diarrhea and nausea (both reported in six [28.6%] patients), vomiting (reported in two [9.5%] patients), anemia, decreased appetite, and constipation (all reported in one [4.8%] patient).
Conclusion: Adavosertib does not have a clinically important effect on QTc prolongation.
Clinicaltrials: GOV: NCT03333824.
Keywords: AZD1775; Adavosertib; Pharmacodynamics; Pharmacokinetics; QT interval; Wee1 inhibitor.
© 2023. The Author(s).
Conflict of interest statement
LDL is a consultant to G1 Therapeutics and 7 Hills Pharma LLC. He also received, through an award to his institution, clinical trial funding for this study from AstraZeneca and other similar clinical trial funding support from the following sponsors: Bristol Myers Squibb, Bayer Pharmaceuticals, and AbbVie. WJE is a consultant for Chimerix Corp. CD, DR, MA, and MN are employees and shareholders of AstraZeneca. LHO was an employee of AstraZeneca at the time of conduct and reporting of this study. LHO is also a shareholder of AstraZeneca. All other authors have declared no conflicts of interest.
Figures


References
-
- Do K, Wilsker D, Ji J, Zlott J, Freshwater T, Kinders RJ, Collins J, Chen AP, Doroshow JH, Kummar S. Phase I study of single-agent AZD1775 (MK-1775), a Wee1 kinase inhibitor, in patients with refractory solid tumors. J Clin Oncol. 2015;33:3409–3415. doi: 10.1200/jco.2014.60.4009. - DOI - PMC - PubMed
-
- Hirai H, Iwasawa Y, Okada M, Arai T, Nishibata T, Kobayashi M, Kimura T, Kaneko N, Ohtani J, Yamanaka K, Itadani H, Takahashi-Suzuki I, Fukasawa K, Oki H, Nambu T, Jiang J, Sakai T, Arakawa H, Sakamoto T, Sagara T, Yoshizumi T, Mizuarai S, Kotani H. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther. 2009;8:2992–3000. doi: 10.1158/1535-7163.mct-09-0463. - DOI - PubMed
-
- Aarts M, Sharpe R, Garcia-Murillas I, Gevensleben H, Hurd MS, Shumway SD, Toniatti C, Ashworth A, Turner NC. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of Wee1. Cancer Discov. 2012;2:524–539. doi: 10.1158/2159-8290.cd-11-0320. - DOI - PubMed
-
- Beck H, Nähse-Kumpf V, Larsen MS, O'Hanlon KA, Patzke S, Holmberg C, Mejlvang J, Groth A, Nielsen O, Syljuåsen RG, Sørensen CS. Cyclin-dependent kinase suppression by Wee1 kinase protects the genome through control of replication initiation and nucleotide consumption. Mol Cell Biol. 2012;32:4226–4236. doi: 10.1128/mcb.00412-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

