Matching-Adjusted Indirect Comparison Between Risankizumab and Ustekinumab for Induction and Maintenance Treatment of Moderately to Severely Active Crohn's Disease
- PMID: 37368103
- PMCID: PMC10427520
- DOI: 10.1007/s12325-023-02546-6
Matching-Adjusted Indirect Comparison Between Risankizumab and Ustekinumab for Induction and Maintenance Treatment of Moderately to Severely Active Crohn's Disease
Abstract
Introduction: Risankizumab (RZB) and ustekinumab (UST), interleukin (IL)-23 and IL-12/23 inhibitors, respectively, are approved treatments for moderately to severely active Crohn's disease (CD); direct comparison between the two is ongoing. We indirectly compared efficacy of RZB versus UST using data from phase 3 trials (RZB: NCT03104413; NCT03105128; NCT03105102; UST: NCT01369329; NCT01369342; NCT01369355).
Methods: Matching-adjusted indirect comparison was conducted using individual patient-level data from RZB trials and published aggregated data from UST trials. During induction, patients received RZB 600 mg intravenous (IV) at weeks 0, 4, and 8 or a single dose of UST 6 mg/kg IV at week 0. During maintenance, patients received RZB 180 or 360 mg subcutaneous (SC) or UST 90 mg SC every 8 or 12 weeks to 52 weeks. Outcomes included proportion of patients achieving Crohn's Disease Activity Index (CDAI) response (decrease of ≥ 100 points or total score < 150) or remission (CDAI ≤ 150) and endoscopic improvement (measured by the Simple Endoscopic Score in CD [SES-CD]; response, ≥ 50% reduction from baseline; remission, SES-CD ≤ 2) following induction/baseline.
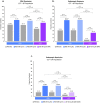
Results: Higher proportions of patients achieved clinical and endoscopic outcomes with RZB vs. UST induction treatment, resulting in significantly (p ≤ 0.05) greater percent differences (95% confidence intervals) between groups for CDAI remission (15% [5%, 25%]) and endoscopic response (26% [13%, 40%]) and remission (9% [0%, 19%]). Following maintenance, rates of CDAI remission were similar (range - 0.3% to - 5.0%) for RZB vs. UST. Differences for endoscopic response and remission ranged from 9.3% to 27.7% and 11.6% to 12.5%, respectively; differences were significant (p < 0.05) for endoscopic response for both doses of RZB compared to UST 12-week dosing.
Conclusions: This indirect comparison demonstrated higher rates of clinical and endoscopic outcomes during induction for RZB compared to UST; CDAI remission following maintenance was comparable. Direct comparisons of RZB and UST are warranted to validate these findings.
Keywords: CDAI; Crohn’s disease; Endoscopic improvement; Matching-adjusted indirect comparison; Risankizumab; Ustekinumab.
Plain language summary
Using individual patient-level data from risankizumab and aggregated data from ustekinumab phase 3 Crohn’s disease trials, we indirectly compared efficacy of risankizumab and ustekinumab to determine whether rates of improvement in disease symptoms (clinical) and endoscopic outcomes differed between treatments. Findings showed that clinical and endoscopic outcomes were more frequently achieved for patients receiving risankizumab versus ustekinumab after induction, while most maintenance outcomes were comparable.
© 2023. The Author(s).
Conflict of interest statement
Marla Dubinsky has received consulting fees from AbbVie, Abivax, Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Ferring, Janssen, Merck, Takeda, Pfizer, Prometheus Biosciences, Prometheus Labs. Christopher Ma has received consulting fees from AbbVie, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol Myers Squibb, Celltrion, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Takeda, Pendopharm, Pfizer, Prometheus Biosciences Inc., Roche, Sanofi; speaker’s fees from AbbVie, Amgen, AVIR Pharma Inc, Alimentiv, Bristol Myers Squibb, Ferring, Fresenius Kabi, Janssen, Takeda, Pendopharm, and Pfizer; royalties from Springer Publishing; research support from Ferring, Pfizer. Tom O’Connell and Marjorie Crowell are employees of Medicus Economics LLC and were contracted by AbbVie for this study. Jenny Griffith, Ezequiel Neimark, and Kristina Kligys are employees of AbbVie and may own stock.
Figures

Similar articles
-
Risankizumab as maintenance therapy for moderately to severely active Crohn's disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial.Lancet. 2022 May 28;399(10340):2031-2046. doi: 10.1016/S0140-6736(22)00466-4. Lancet. 2022. PMID: 35644155 Clinical Trial.
-
Efficacy and safety of 48 weeks of guselkumab for patients with Crohn's disease: maintenance results from the phase 2, randomised, double-blind GALAXI-1 trial.Lancet Gastroenterol Hepatol. 2024 Feb;9(2):133-146. doi: 10.1016/S2468-1253(23)00318-7. Epub 2023 Dec 14. Lancet Gastroenterol Hepatol. 2024. PMID: 38104569 Clinical Trial.
-
Risankizumab in patients with moderate to severe Crohn's disease: an open-label extension study.Lancet Gastroenterol Hepatol. 2018 Oct;3(10):671-680. doi: 10.1016/S2468-1253(18)30233-4. Epub 2018 Jul 25. Lancet Gastroenterol Hepatol. 2018. PMID: 30056030 Clinical Trial.
-
One-Year Efficacy of Guselkumab Versus Advanced Therapies for the Treatment of Moderately to Severely Active Crohn's Disease: A Network Meta-Analysis.Adv Ther. 2025 Jun;42(6):2708-2727. doi: 10.1007/s12325-025-03183-x. Epub 2025 May 6. Adv Ther. 2025. PMID: 40327280 Free PMC article. Review.
-
Anti-IL-12/23p40 antibodies for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012804. doi: 10.1002/14651858.CD012804.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828765 Free PMC article.
Cited by
-
New Interleukin-23 Antagonists' Use in Crohn's Disease.Pharmaceuticals (Basel). 2025 Mar 22;18(4):447. doi: 10.3390/ph18040447. Pharmaceuticals (Basel). 2025. PMID: 40283885 Free PMC article. Review.
-
Immunity in digestive diseases: new drugs for inflammatory bowel disease treatment-insights from Phase II and III trials.J Gastroenterol. 2024 Sep;59(9):761-787. doi: 10.1007/s00535-024-02130-x. Epub 2024 Jul 9. J Gastroenterol. 2024. PMID: 38980426 Free PMC article. Review.
-
Psoriasis and inflammatory bowel disease: concomitant IMID or paradoxical therapeutic effect? A scoping review on anti-IL-12/23 and anti-IL-23 antibodies.Therap Adv Gastroenterol. 2024 Nov 21;17:17562848241299564. doi: 10.1177/17562848241299564. eCollection 2024. Therap Adv Gastroenterol. 2024. PMID: 39575159 Free PMC article.
-
Ustekinumab in the Treatment of Crohn's Disease-A Narrative Review on Clinical Efficacy and Safety Profile.Pharmacy (Basel). 2025 May 21;13(3):73. doi: 10.3390/pharmacy13030073. Pharmacy (Basel). 2025. PMID: 40407511 Free PMC article. Review.
-
Comparing the efficacy and safety of safinamide with rasagiline in China Parkinson's disease patients with a matching adjusted indirect comparison.Sci Rep. 2025 Mar 26;15(1):10502. doi: 10.1038/s41598-025-94960-9. Sci Rep. 2025. PMID: 40140610 Free PMC article. Clinical Trial.
References
-
- Mehta F. Economic implications of inflammatory bowel disease and its management. Am J Manag Care. 2016;22:10. - PubMed
-
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

