CD47 blockade ameliorates autoimmune vasculitis via efferocytosis of neutrophil extracellular traps
- PMID: 37368493
- PMCID: PMC10445685
- DOI: 10.1172/jci.insight.167486
CD47 blockade ameliorates autoimmune vasculitis via efferocytosis of neutrophil extracellular traps
Abstract
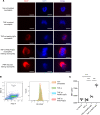
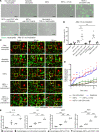
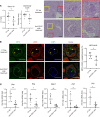
Neutrophil extracellular trap (NET) formation contributes to immune defense and is a distinct form of cell death. Excessive NET formation is found in patients with anti-neutrophil cytoplasmic antibody-associated (ANCA-associated) vasculitis (AAV), contributing to disease progression. The clearance of dead cells by macrophages, a process known as efferocytosis, is regulated by the CD47-mediated "don't eat me" signal. Hence, we hypothesized that pathogenic NETs in AAV escape from efferocytosis via the CD47 signaling pathway, resulting in the development of necrotizing vasculitis. Immunostaining for CD47 in human renal tissues revealed high CD47 expression in crescentic glomerular lesions of patients with AAV. In ex vivo studies, ANCA-induced netting neutrophils increased the expression of CD47 with the reduction of efferocytosis. After efferocytosis, macrophages displayed proinflammatory phenotypes. The blockade of CD47 in spontaneous crescentic glomerulonephritis-forming/Kinjoh (SCG/Kj) mice ameliorated renal disease and reduced myeloperoxidase-ANCA (MPO-ANCA) titers with a reduction in NET formation. Thus, CD47 blockade would protect against developing glomerulonephritis in AAV via restored efferocytosis of ANCA-induced NETs.
Keywords: Autoimmunity; Nephrology; Vasculitis.
Conflict of interest statement
Figures


Similar articles
-
Vasculitis and crescentic glomerulonephritis in a newly established congenic mouse strain derived from ANCA-associated vasculitis-prone SCG/Kj mice.Autoimmunity. 2019 Aug-Sep;52(5-6):208-219. doi: 10.1080/08916934.2019.1658191. Epub 2019 Sep 2. Autoimmunity. 2019. PMID: 31476889
-
Myeloperoxidase anti-neutrophil cytoplasmic antibody affinity is associated with the formation of neutrophil extracellular traps in the kidney and vasculitis activity in myeloperoxidase anti-neutrophil cytoplasmic antibody-associated microscopic polyangiitis.Nephrology (Carlton). 2016 Jul;21(7):624-9. doi: 10.1111/nep.12736. Nephrology (Carlton). 2016. PMID: 26833773 Free PMC article.
-
Anti-neutrophil extracellular trap antibody in a patient with relapse of anti-neutrophil cytoplasmic antibody-associated vasculitis: a case report.BMC Nephrol. 2018 Jun 22;19(1):145. doi: 10.1186/s12882-018-0953-y. BMC Nephrol. 2018. PMID: 29929470 Free PMC article.
-
New advances in the pathogenesis of ANCA-associated vasculitides.Clin Exp Rheumatol. 2009 Jan-Feb;27(1 Suppl 52):S108-14. Clin Exp Rheumatol. 2009. PMID: 19646356 Review.
-
Neutrophil Extracellular Traps: A Potential Therapeutic Target in MPO-ANCA Associated Vasculitis?Front Immunol. 2021 Mar 15;12:635188. doi: 10.3389/fimmu.2021.635188. eCollection 2021. Front Immunol. 2021. PMID: 33790907 Free PMC article. Review.
Cited by
-
The involvement of NETs in ANCA-associated vasculitis.Front Immunol. 2023 Sep 14;14:1261151. doi: 10.3389/fimmu.2023.1261151. eCollection 2023. Front Immunol. 2023. PMID: 37781373 Free PMC article. Review.
-
Transcriptional analysis of efferocytosis in mouse skin wounds.bioRxiv [Preprint]. 2024 Aug 13:2024.08.12.607219. doi: 10.1101/2024.08.12.607219. bioRxiv. 2024. PMID: 39185146 Free PMC article. Preprint.
-
Cell autonomous functions of CD47 in regulating cellular plasticity and metabolic plasticity.Cell Death Differ. 2024 Oct;31(10):1255-1266. doi: 10.1038/s41418-024-01347-w. Epub 2024 Jul 23. Cell Death Differ. 2024. PMID: 39039207 Free PMC article. Review.
-
Neutrophils: a Central Point of Interaction Between Immune Cells and Nonimmune Cells in Rheumatoid Arthritis.Clin Rev Allergy Immunol. 2025 Mar 28;68(1):34. doi: 10.1007/s12016-025-09044-3. Clin Rev Allergy Immunol. 2025. PMID: 40148714 Review.
-
Beyond cancer: The potential application of CD47-based therapy in non-cancer diseases.Acta Pharm Sin B. 2025 Feb;15(2):757-791. doi: 10.1016/j.apsb.2024.11.018. Epub 2024 Nov 28. Acta Pharm Sin B. 2025. PMID: 40177549 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

