Homoleptic Fe(III) and Fe(IV) Complexes of a Dianionic C3-Symmetric Scorpionate
- PMID: 37369076
- PMCID: PMC10336972
- DOI: 10.1021/acs.inorgchem.3c00871
Homoleptic Fe(III) and Fe(IV) Complexes of a Dianionic C3-Symmetric Scorpionate
Abstract
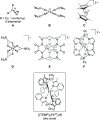
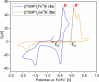
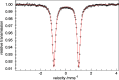
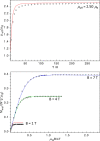
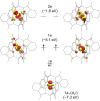
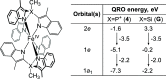
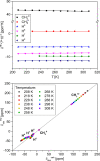
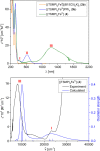
High-valent iron species have been implicated as key intermediates in catalytic oxidation reactions, both in biological and synthetic systems. Many heteroleptic Fe(IV) complexes have now been prepared and characterized, especially using strongly π-donating oxo, imido, or nitrido ligands. On the other hand, homoleptic examples are scarce. Herein, we investigate the redox chemistry of iron complexes of the dianonic tris-skatylmethylphosphonium (TSMP2-) scorpionate ligand. One-electron oxidation of the tetrahedral, bis-ligated [(TSMP)2FeII]2- leads to the octahedral [(TSMP)2FeIII]-. The latter undergoes thermal spin-cross-over both in the solid state and solution, which we characterize using superconducting quantum inference device (SQUID), Evans method, and paramagnetic nuclear magnetic resonance spectroscopy. Furthermore, [(TSMP)2FeIII]- can be reversibly oxidized to the stable high-valent [(TSMP)2FeIV]0 complex. We use a variety of electrochemical, spectroscopic, and computational techniques as well as SQUID magnetometry to establish a triplet (S = 1) ground state with a metal-centered oxidation and little spin delocalization on the ligand. The complex also has a fairly isotropic g-tensor (giso = 1.97) combined with a positive zero-field splitting (ZFS) parameter D (+19.1 cm-1) and very low rhombicity, in agreement with quantum chemical calculations. This thorough spectroscopic characterization contributes to a general understanding of octahedral Fe(IV) complexes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
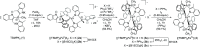








References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

