Efficacy, tolerability, and endometrial safety of ospemifene compared with current therapies for the treatment of vulvovaginal atrophy: a systematic literature review and network meta-analysis
- PMID: 37369079
- PMCID: PMC10389189
- DOI: 10.1097/GME.0000000000002211
Efficacy, tolerability, and endometrial safety of ospemifene compared with current therapies for the treatment of vulvovaginal atrophy: a systematic literature review and network meta-analysis
Abstract
Importance: Ospemifene is a novel selective estrogen receptor modulator developed for the treatment of moderate to severe postmenopausal vulvovaginal atrophy (VVA).
Objective: The aim of the study is to perform a systematic literature review (SLR) and network meta-analysis (NMA) to assess the efficacy and safety of ospemifene compared with other therapies used in the treatment of VVA in North America and Europe.
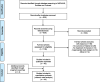
Evidence review: Electronic database searches were conducted in November 2021 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Randomized or nonrandomized controlled trials targeting postmenopausal women with moderate to severe dyspareunia and/or vaginal dryness and involving ospemifene or at least one VVA local treatment were considered. Efficacy data included changes from baseline in superficial and parabasal cells, vaginal pH, and the most bothersome symptom of vaginal dryness or dyspareunia, as required for regulatory approval. Endometrial outcomes were endometrial thickness and histologic classifications, including endometrial polyp, hyperplasia, and cancer. For efficacy and safety outcomes, a Bayesian NMA was performed. Endometrial outcomes were compared in descriptive analyses.
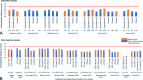
Findings: A total of 44 controlled trials met the eligibility criteria ( N = 12,637 participants). Network meta-analysis results showed that ospemifene was not statistically different from other active therapies in most efficacy and safety results. For all treatments, including ospemifene, the posttreatment endometrial thickness values (up to 52 wk of treatment) were under the recognized clinical threshold value of 4 mm for significant risk of endometrial pathology. Specifically, for women treated with ospemifene, endometrial thickness ranged between 2.1 and 2.3 mm at baseline and 2.5 and 3.2 mm after treatment. No cases of endometrial carcinoma or hyperplasia were observed in ospemifene trials, nor polyps with atypical hyperplasia or cancer after up to 52 weeks of treatment.
Conclusions and relevance: Ospemifene is an efficacious, well-tolerated, and safe therapeutic option for postmenopausal women with moderate to severe symptoms of VVA. Efficacy and safety outcomes with ospemifene are similar to other VVA therapies in North America and Europe.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The North American Menopause Society.
Conflict of interest statement
Financial disclosure/conflicts of interest: C.B. is a partner at PeriPharm, Inc, and A.C. and C.R. are employees at PeriPharm, Inc, a company that has served as a consultant to Duchesnay, Inc, and has received funding from Duchesnay, Inc. A.F. and D.B. have served as consultants for Duchesnay, Inc. D.B. currently receives funding from AbbVie, Bayer, BioSyent, Duchesnay, Merck, Organon, Pfizer, and Searchlight. R.M. is an employee at Duchesnay, Inc. J.A.S. receives grant/research support from the following: AbbVie, Bayer Healthcare, Dare Bioscience, Enteris BioPharma, Mylan/Viatris, Myovant Sciences, ObsEva, and Viveve Medical; is on consulting/advisory boards of the following: Bayer Healthcare, Besins Healthcare, California Institute of Integral Studies (CIIS), Dare Bioscience, DEKA M.E.L.A S.r.l., Duchesnay, Inc, Femasys, KaNDy/NeRRe Therapeutics, Khyria, Madorra, Mitsubishi Tanabe Pharma Development America, QUE Oncology, Scynexis, Inc, Sprout Pharmaceuticals, Vella Bioscience; serves on the speaker's bureaus of the following: Mayne Pharma, Myovant Sciences, Pfizer, Pharmavite, Scynexis, and TherapeuticsMD; and is a stockholder (direct purchase) in Sermonix Pharmaceuticals.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

