Pluripotent stem cell-derived model of the post-implantation human embryo
- PMID: 37369347
- PMCID: PMC10584688
- DOI: 10.1038/s41586-023-06368-y
Pluripotent stem cell-derived model of the post-implantation human embryo
Erratum in
-
Publisher Correction: Pluripotent stem cell-derived model of the post-implantation human embryo.Nature. 2023 Sep;621(7977):E30. doi: 10.1038/s41586-023-06524-4. Nature. 2023. PMID: 37592011 Free PMC article. No abstract available.
Abstract
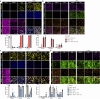
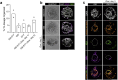
The human embryo undergoes morphogenetic transformations following implantation into the uterus, but our knowledge of this crucial stage is limited by the inability to observe the embryo in vivo. Models of the embryo derived from stem cells are important tools for interrogating developmental events and tissue-tissue crosstalk during these stages1. Here we establish a model of the human post-implantation embryo, a human embryoid, comprising embryonic and extraembryonic tissues. We combine two types of extraembryonic-like cell generated by overexpression of transcription factors with wild-type embryonic stem cells and promote their self-organization into structures that mimic several aspects of the post-implantation human embryo. These self-organized aggregates contain a pluripotent epiblast-like domain surrounded by extraembryonic-like tissues. Our functional studies demonstrate that the epiblast-like domain robustly differentiates into amnion, extraembryonic mesenchyme and primordial germ cell-like cells in response to bone morphogenetic protein cues. In addition, we identify an inhibitory role for SOX17 in the specification of anterior hypoblast-like cells2. Modulation of the subpopulations in the hypoblast-like compartment demonstrates that extraembryonic-like cells influence epiblast-like domain differentiation, highlighting functional tissue-tissue crosstalk. In conclusion, we present a modular, tractable, integrated3 model of the human embryo that will enable us to probe key questions of human post-implantation development, a critical window during which substantial numbers of pregnancies fail.
© 2023. The Author(s).
Conflict of interest statement
The authors are inventors on the following patents: (1) Patent applicant: Caltech; inventors: M.Z.-G., B.S. and V.J.; application number: 17/692,790; specific aspect of the manuscript covered in patent application: reconstructing human early embryogenesis in vitro with pluripotent stem cells. (2) Patent applicant: Caltech and Cambridge Enterprise Limited; inventors: M.Z.-G., G.A. and C.H.; application number: 63/397,630; specific aspect of the manuscript covered in patent application: synthetic embryos. (3) Patent applicant: Caltech and Cambridge Enterprise Limited; inventors: M.Z.-G., B.W. and C.G.; application number: 63/403,684; specific aspect of the manuscript covered in patent application: stem cell-derived model of the human embryo.
Figures












Comment in
-
Dawn of development: Exploring early human embryogenesis using stem cells.Cell Stem Cell. 2023 Aug 3;30(8):1006-1007. doi: 10.1016/j.stem.2023.07.009. Cell Stem Cell. 2023. PMID: 37541206
-
Models of the early human embryo.Nat Biotechnol. 2023 Aug;41(8):1072. doi: 10.1038/s41587-023-01920-7. Nat Biotechnol. 2023. PMID: 37568022 No abstract available.
-
Stem cells used to model a two-week-old human embryo.Nature. 2023 Oct;622(7983):469-470. doi: 10.1038/d41586-023-03150-y. Nature. 2023. PMID: 37848524 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

