European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer - Update 2023
- PMID: 37369376
- PMCID: PMC10359596
- DOI: 10.1136/ijgc-2023-004486
European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer - Update 2023
Abstract
Background: As part of its mission to improve the quality of care for women with gynecological cancers across Europe, the European Society of Gynaecological Oncology (ESGO) first published in 2017 evidence-based guidelines for the management of patients with vulvar cancer.
Objective: To update the ESGO guidelines based on the new evidence addressing the management of vulvar cancer and to cover new topics in order to provide comprehensive guidelines on all relevant issues of diagnosis and treatment of vulvar cancer.
Methods: The ESGO Council nominated an international development group comprised of practicing clinicians who provide care to vulvar cancer patients and have demonstrated leadership through their expertize in clinical care and research, national and international engagement and profile as well as dedication to the topics addressed to serve on the expert panel (18 experts across Europe). To ensure that the statements were evidence-based, new data identified from a systematic search were reviewed and critically appraised. In the absence of any clear scientific evidence, judgment was based on the professional experience and consensus of the international development group. Prior to publication, the guidelines were reviewed by 206 international practitioners in cancer care delivery and patient representatives.
Results: The updated guidelines cover comprehensively diagnosis and referral, staging, pathology, pre-operative investigations, surgical management (local treatment, groin treatment, sentinel lymph node procedure, reconstructive surgery), (chemo)radiotherapy, systemic treatment, treatment of recurrent disease (vulvar, inguinal, pelvic, and distant recurrences), and follow-up. Management algorithms are also defined.
Keywords: Vulvar and Vaginal Cancer.
© IGCS and ESGO 2023. Re-use permitted under CC BY-NC. No commercial re-use. Published by BMJ.
Conflict of interest statement
Competing interests: SM has reported advisory boards for AbbVie, AstraZeneca, Clovis, Eisai, GlaxoSmithKline, Hubro, MEdac, MSD, Novartis, Nykode, Novartis, Olympus, PharmaMar, Pfizer, Roche, Sensor Kinesis, Teva, Tesaro, and grants for travelling from AbbVie, AstraZeneca, Clovis, Eisai, GlaxoSmithKline, Hubro, MEdac, MSD, Novartis, Nykode, Olympus, PharmaMar, Pfizer, Roche, Sensor Kinesis, Teva, Tesaro. AR has reported institutional grants from Eisai, PharmaMar, Roche, speaker’s bureau for AstraZeneca, MSD, GlaxoSmithKline, PharmaMar, Clovis, advisory boards for AstraZeneca, Eisai, GlaxoSmithKline, PharmaMar, Clovis, and grants for travelling from AstraZeneca, GlaxoSmithKline, Clovis, and PharmaMar. AS has reported grants for travelling from Elekta, Stiftung Filantropie Österreich, and Medizinische Universität Wien. AT has reported advisory boards for MSD. LW has reported funding from MEdac Oncology, Roche Diagnostics, Hamburger KG, DKH, honoraria from Roche, Tesaro, Pfizer, GlaxoSmithKline, GynOnko Update, AstraZeneca, Teva, Omniamed, Promedicis, MSD, Eisai, Seagen, and advisory boards for MSD, GlaxoSmithKline, Roche, Eisai, and Seagen. MHMO, FP, PB, MRM, DF, CLC, EG, GG, SL, EU, VV, AvdZ, DZ, GFZ, and IZ have reported no conflicts of interest.
Figures

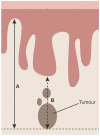

References
-
- World Health Organization . GLOBOCAN 2020: estimated cancer incidence, mortality and prevalence worldwide in 2020. 2022. Available: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_... [Accessed 08 Jun 2022].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

