βII-Spectrin Is Required for Synaptic Positioning during Retinal Development
- PMID: 37369589
- PMCID: PMC10359034
- DOI: 10.1523/JNEUROSCI.0063-23.2023
βII-Spectrin Is Required for Synaptic Positioning during Retinal Development
Abstract
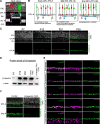
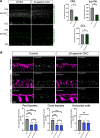
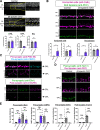
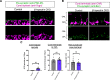
Neural circuit assembly is a multistep process where synaptic partners are often born at distinct developmental stages, and yet they must find each other and form precise synaptic connections with one another. This developmental process often relies on late-born neurons extending their processes to the appropriate layer to find and make synaptic connections to their early-born targets. The molecular mechanism responsible for the integration of late-born neurons into an emerging neural circuit remains unclear. Here, we uncovered a new role for the cytoskeletal protein βII-spectrin in properly positioning presynaptic and postsynaptic neurons to the developing synaptic layer. Loss of βII-spectrin disrupts retinal lamination, leads to synaptic connectivity defects, and results in impaired visual function in both male and female mice. Together, these findings highlight a new function of βII-spectrin in assembling neural circuits in the mouse outer retina.SIGNIFICANCE STATEMENT Neurons that assemble into a functional circuit are often integrated at different developmental time points. However, the molecular mechanism that guides the precise positioning of neuronal processes to the correct layer for synapse formation is relatively unknown. Here, we show a new role for the cytoskeletal scaffolding protein, βII-spectrin in the developing retina. βII-spectrin is required to position presynaptic and postsynaptic neurons to the nascent synaptic layer in the mouse outer retina. Loss of βII-spectrin disrupts positioning of neuronal processes, alters synaptic connectivity, and impairs visual function.
Keywords: neurodevelopment; retina; spectrins; synapse.
Copyright © 2023 the authors.
Figures


References
-
- Bennett V, Lorenzo DN (2013) Spectrin- and ankyrin-based membrane domains and the evolution of vertebrates. Curr Top Membr 72:1–37. - PubMed
-
- Bennett V, Lorenzo DN (2016) An adaptable spectrin/ankyrin-based mechanism for long-range organization of plasma membranes in vertebrate tissues. Curr Top Membr 77:143–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
