Extracellular matrix-derived mechanical force governs breast cancer cell stemness and quiescence transition through integrin-DDR signaling
- PMID: 37369642
- PMCID: PMC10300038
- DOI: 10.1038/s41392-023-01453-0
Extracellular matrix-derived mechanical force governs breast cancer cell stemness and quiescence transition through integrin-DDR signaling
Abstract
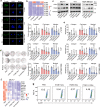
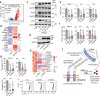
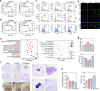
The extracellular matrix (ECM) serves as signals that regulate specific cell states in tumor tissues. Increasing evidence suggests that extracellular biomechanical force signals are critical in tumor progression. In this study, we aimed to explore the influence of ECM-derived biomechanical force on breast cancer cell status. Experiments were conducted using 3D collagen, fibrinogen, and Matrigel matrices to investigate the role of mechanical force in tumor development. Integrin-cytoskeleton-AIRE and DDR-STAT signals were examined using RNA sequencing and western blotting. Data from 1358 patients and 86 clinical specimens were used for ECM signature-prognosis analysis. Our findings revealed that ECM-derived mechanical force regulated tumor stemness and cell quiescence in breast cancer cells. A mechanical force of ~45 Pa derived from the extracellular substrate activated integrin β1/3 receptors, stimulating stem cell signaling pathways through the cytoskeleton/AIRE axis and promoting tumorigenic potential and stem-like phenotypes. However, excessive mechanical force (450 Pa) could drive stem-like cancer cells into a quiescent state, with the removal of mechanical forces leading to vigorous proliferation in quiescent cancer stem cells. Mechanical force facilitated cell cycle arrest to induce quiescence, dependent on DDR2/STAT1/P27 signaling. Therefore, ECM-derived mechanical force governs breast cancer cell status and proliferative characteristics through stiffness alterations. We further established an ECM signature based on the fibrinogen/fibronectin/vitronectin/elastin axis, which efficiently predicts patient prognosis in breast cancer. Our findings highlight the vital role of ECM-derived mechanical force in governing breast cancer cell stemness/quiescence transition and suggest the novel use of ECM signature in predicting the clinical prognosis of breast cancer.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

