Subcellular second messenger networks drive distinct repellent-induced axon behaviors
- PMID: 37369692
- PMCID: PMC10300027
- DOI: 10.1038/s41467-023-39516-z
Subcellular second messenger networks drive distinct repellent-induced axon behaviors
Abstract
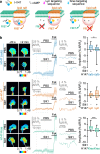
Second messengers, including cAMP, cGMP and Ca2+ are often placed in an integrating position to combine the extracellular cues that orient growing axons in the developing brain. This view suggests that axon repellents share the same set of cellular messenger signals and that axon attractants evoke opposite cAMP, cGMP and Ca2+ changes. Investigating the confinement of these second messengers in cellular nanodomains, we instead demonstrate that two repellent cues, ephrin-A5 and Slit1, induce spatially segregated signals. These guidance molecules activate subcellular-specific second messenger crosstalk, each signaling network controlling distinct axonal morphology changes in vitro and pathfinding decisions in vivo.
© 2023. The Author(s).
Conflict of interest statement
O.R. and X.N. hold patents describing SpiCee and SponGee. The remaining authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

