Timing of interval debulking surgery and postoperative chemotherapy after neoadjuvant chemotherapy in advanced epithelial ovarian cancer: a multicenter real-world study
- PMID: 37370087
- PMCID: PMC10294495
- DOI: 10.1186/s13048-023-01164-8
Timing of interval debulking surgery and postoperative chemotherapy after neoadjuvant chemotherapy in advanced epithelial ovarian cancer: a multicenter real-world study
Abstract
Background: To investigate the prognostic relevance of the time to interval debulking surgery (TTS) and the time to postoperative adjuvant chemotherapy (TTC) after the completion of neoadjuvant chemotherapy (NACT).
Methods: A retrospective real-word study included 658 patients with histologically confirmed advanced epithelial ovarian cancer who received NACT at seven tertiary hospitals in China from June 2008 to June 2020. TTS was defined as the time interval from the completion of NACT to the time of interval debulking surgery (IDS). TTC was defined as the time interval from the completion of NACT to the initiation of postoperative adjuvant chemotherapy (PACT).
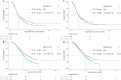
Results: The median TTS and TTC were 25 (IQR, 20-29) and 40 (IQR, 33-49) days, respectively. Patients with TTS > 25 days were older (55 vs. 53 years, P = 0.012) and received more NACT cycles (median, 3 vs. 2, P = 0.002). Similar results were observed in patients with TTC > 40 days. In the multivariate analyses, TTS and TTC were not associated with PFS when stratified by median, quartile, or integrated as continuous variables (all P > 0.05). However, TTS and TTC were significantly associated with worse OS when stratified by median (P = 0.018 and 0.018, respectively), quartile (P = 0.169, 0.014, 0.027 and 0.012, 0.001, 0.033, respectively), or integrated as continuous variables (P = 0.018 and 0.011, respectively). Similarly, increasing TTS and TTC intervals were associated with a higher risk of death (Ptrend = 0.016 and 0.031, respectively) but not with recurrence (Ptrend = 0.103 and 0.381, respectively).
Conclusion: The delays of IDS and PACT after the completion of NACT have adverse impacts on OS but no impacts on PFS, which indicates that reducing delays of IDS and PACT might ameliorate the outcomes of ovarian cancer patients treated with NACT.
Keywords: Advanced epithelial ovarian cancer; Neoadjuvant chemotherapy; Prognosis; Time to interval debulking surgery; Time to postoperative adjuvant chemotherapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer Statistics. 2021. CA: a cancer journal for clinicians. 2021;71:7–33. - PubMed
-
- Ranking. (Ovary), estimated number of new cases and deaths in 2020, all ages. https://gco.iarc.fr/today/online-analysis-map.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

