Unique features of the arterial blood-brain barrier
- PMID: 37370096
- PMCID: PMC10294539
- DOI: 10.1186/s12987-023-00450-3
Unique features of the arterial blood-brain barrier
Abstract
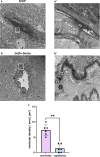
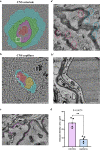
CNS vasculature differs from vascular networks of peripheral organs by its ability to tightly control selective material exchange across capillary barriers. Capillary permeability is mostly defined by unique cellular components of the endothelium. While capillaries are extensively investigated, the barrier properties of larger vessels are understudied. Here, we investigate barrier properties of CNS arterial walls. Using tracer challenges and various imaging modalities, we discovered that at the mouse cortex, the arterial barrier does not reside at the classical level of the endothelium. The arterial wall's unique permeability acts bi-directionally; CSF substances travel along the glymphatic path and can penetrate from the peri-vascular space through arteriolar walls towards the lumen. We found that caveolae vesicles in arteriole endothelial are functional transcytosis machinery components, and that a similar mechanism is evident in the human brain. Our discoveries highlight vascular heterogeneity investigations as a potent approach to uncover new barrier mechanisms.
Keywords: Arterial berrier; BBB; Super-resolution; Transcytosis; dSTORM.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

