The Use of Intravenous Fosfomycin in Clinical Practice: A 5-Year Retrospective Study in a Tertiary Hospital in Italy
- PMID: 37370290
- PMCID: PMC10295113
- DOI: 10.3390/antibiotics12060971
The Use of Intravenous Fosfomycin in Clinical Practice: A 5-Year Retrospective Study in a Tertiary Hospital in Italy
Abstract
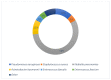
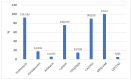
Fosfomycin in intravenous (IV) formulation has re-emerged as a valuable tool in the treatment of multi-drug resistant (MDR) and extensively drug-resistant (XDR) infections because of its broad spectrum of antibacterial action and pharmacokinetic characteristics. This retrospective study aimed to evaluate how fosfomycin was used in patients admitted to the Polyclinic of Palermo between January 2017 and July 2022. Clinical indications, therapeutic associations, clinical outcomes, and any side effects were analyzed. Intravenous fosfomycin was used in 343 patients, 63% male, with a mean age of 68 years (range 15-95). Urinary tract infections (UTIs) and hospital-acquired pneumonia (HAP) were the main indications for treatment (19% and 18% of the total cases, respectively), followed by skin and soft tissue infections and sepsis. IV fosfomycin was administered in combination with other antibacterial agents, the most common of which were ceftazidime/avibactam (35%), meropenem (17%), and colistin (14%). Nineteen patients received it as monotherapy for UTIs. About 66% had resolution of the infectious process with clinical remission (cure or discharge). Electrolyte disturbances occurred in 2.6% and gastrointestinal symptoms occurred in 2.9%. The data showed that IV fosfomycin is a safe and effective therapeutic option in the treatment of infections with multidrug-resistant microorganisms.
Keywords: antimicrobials; fosfomycin; gram-negative; retrospective study.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

