Antibiotic Treatment Induces Long-Lasting Effects on Gut Microbiota and the Enteric Nervous System in Mice
- PMID: 37370319
- PMCID: PMC10295661
- DOI: 10.3390/antibiotics12061000
Antibiotic Treatment Induces Long-Lasting Effects on Gut Microbiota and the Enteric Nervous System in Mice
Abstract
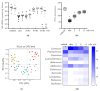
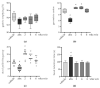
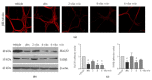
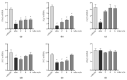
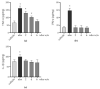
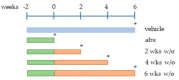
The side effects of antibiotic treatment directly correlate with intestinal dysbiosis. However, a balanced gut microbiota supports the integrity of the enteric nervous system (ENS), which controls gastrointestinal neuromuscular functions. In this study, we investigated the long-term effects of antibiotic-induced microbial dysbiosis on the ENS and the impact of the spontaneous re-establishment of the gut microbiota on gastrointestinal functions. C57BL/6J mice were treated daily for two weeks with antibiotics. After 0-6 weeks of antibiotics wash-out, we determined (a) gut microbiota composition, (b) gastrointestinal motility, (c) integrity of the ENS, (d) neurochemical code, and (e) inflammation. Two weeks of antibiotic treatment significantly altered gut microbial composition; the genera Clostridium, Lachnoclostridium, and Akkermansia did not regain their relative abundance following six weeks of antibiotic discontinuation. Mice treated with antibiotics experienced delayed gastrointestinal transit and altered expression of neuronal markers. The anomalies of the ENS persisted for up to 4 weeks after the antibiotic interruption; the expression of neuronal HuC/D, glial-derived neurotrophic factor (Gdnf), and nerve growth factor (Ngf) mRNA transcripts did not recover. In this study, we strengthened the idea that antibiotic-induced gastrointestinal dysmotility directly correlates with gut dysbiosis as well as structural and functional damage to the ENS.
Keywords: antibiotic; enteric nervous system; gastrointestinal motility disorder; gut microbiota; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice.Br J Pharmacol. 2017 Oct;174(20):3623-3639. doi: 10.1111/bph.13965. Epub 2017 Aug 30. Br J Pharmacol. 2017. PMID: 28755521 Free PMC article.
-
Neonatal antibiotics have long term sex-dependent effects on the enteric nervous system.J Physiol. 2022 Oct;600(19):4303-4323. doi: 10.1113/JP282939. Epub 2022 Sep 9. J Physiol. 2022. PMID: 36082768 Free PMC article.
-
Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells.Mol Cell Neurosci. 2015 Sep;68:24-35. doi: 10.1016/j.mcn.2015.03.018. Epub 2015 Mar 27. Mol Cell Neurosci. 2015. PMID: 25823690
-
The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system.J Neuroendocrinol. 2019 May;31(5):e12684. doi: 10.1111/jne.12684. Epub 2019 Feb 1. J Neuroendocrinol. 2019. PMID: 30614568 Review.
-
Microbial Modulation of the Development and Physiology of the Enteric Nervous System.Trends Microbiol. 2021 Aug;29(8):686-699. doi: 10.1016/j.tim.2020.11.007. Epub 2020 Dec 9. Trends Microbiol. 2021. PMID: 33309188 Review.
Cited by
-
Long-Term Implicit Epigenetic Stress Information in the Enteric Nervous System and its Contribution to Developing and Perpetuating IBS.Curr Neuropharmacol. 2024;22(13):2100-2112. doi: 10.2174/1570159X22666240507095700. Curr Neuropharmacol. 2024. PMID: 38726788 Free PMC article. Review.
-
Factors associated with frozen shoulder in adults: a retrospective study.BMC Musculoskelet Disord. 2024 Jun 26;25(1):493. doi: 10.1186/s12891-024-07614-8. BMC Musculoskelet Disord. 2024. PMID: 38926699 Free PMC article.
-
The Impact of Antibiotic Therapy on Intestinal Microbiota: Dysbiosis, Antibiotic Resistance, and Restoration Strategies.Antibiotics (Basel). 2025 Apr 3;14(4):371. doi: 10.3390/antibiotics14040371. Antibiotics (Basel). 2025. PMID: 40298495 Free PMC article. Review.
-
Can the Evidence-Based Use of Probiotics (Notably Saccharomyces boulardii CNCM I-745 and Lactobacillus rhamnosus GG) Mitigate the Clinical Effects of Antibiotic-Associated Dysbiosis?Adv Ther. 2024 Mar;41(3):901-914. doi: 10.1007/s12325-024-02783-3. Epub 2024 Jan 30. Adv Ther. 2024. PMID: 38286962 Free PMC article.
-
Nutritional Strategies for Preterm Neonates and Preterm Neonates Undergoing Surgery: New Insights for Practice and Wrong Beliefs to Uproot.Nutrients. 2024 May 31;16(11):1719. doi: 10.3390/nu16111719. Nutrients. 2024. PMID: 38892652 Free PMC article. Review.
References
-
- Vicentini F.A., Keenan C.M., Wallace L.E., Woods C., Cavin J.-B., Flockton A.R., Macklin W.B., Belkind-Gerson J., Hirota S.A., Sharkey K.A. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. 2021;9:1–24. doi: 10.1186/s40168-021-01165-z. - DOI - PMC - PubMed
-
- Brun P., Giron M.C., Qesari M., Porzionato A., Caputi V., Zoppellaro C., Banzato S., Grillo A.R., Spagnol L., De Caro R., et al. Toll-Like Receptor 2 Regulates Intestinal Inflammation by Controlling Integrity of the Enteric Nervous System. Gastroenterology. 2013;145:1323–1333. doi: 10.1053/j.gastro.2013.08.047. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

