3,3'-Diindolylmethane (DIM): A Potential Therapeutic Agent against Cariogenic Streptococcus mutans Biofilm
- PMID: 37370336
- PMCID: PMC10295630
- DOI: 10.3390/antibiotics12061017
3,3'-Diindolylmethane (DIM): A Potential Therapeutic Agent against Cariogenic Streptococcus mutans Biofilm
Abstract
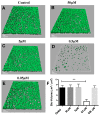
Indole, a metabolite of the amino acid tryptophan, has been proven to act as a signal molecule in bacteria, acting in different aspects of biofilm formation. The oral biofilm is a type of biofilm that has consequences for human health. It is a complex, three-dimensional structure that develops on the surface of teeth via the attachment of primary microbial colonizers. Many oral infections are caused by an imbalance occurring in the microorganisms naturally found in oral biofilms and are considered major public health concerns. In this study, we test the effect of a natural bis-indole, 3,3'-Diindolylmethane (DIM), in mitigating the pathogenicity of the oral biofilm inhabiting bacterium Streptococcus mutans, a bacterium that is considered to be a principal etiological agent in dental caries. Our study found that DIM was able to attenuate S. mutans biofilm formation by 92%. Additionally, treatment with DIM lowered extracellular polymeric substance (EPS) production and decreased its durability significantly under acidic conditions. Therefore, the anti-biofilm and anti-virulence properties of DIM against S. mutans bacteria in an "oral setting" provides evidence for its usefulness in reducing biofilm formation and potentially for caries attenuation.
Keywords: 3,3′-Diindolylmethane; S. mutans; biofilm; caries; resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

