Staphylococcus aureus Small-Colony Variants from Airways of Adult Cystic Fibrosis Patients as Precursors of Adaptive Antibiotic-Resistant Mutations
- PMID: 37370388
- PMCID: PMC10294822
- DOI: 10.3390/antibiotics12061069
Staphylococcus aureus Small-Colony Variants from Airways of Adult Cystic Fibrosis Patients as Precursors of Adaptive Antibiotic-Resistant Mutations
Abstract
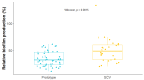
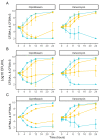
Prototypic Staphylococcus aureus and their small-colony variants (SCVs) are predominant in cystic fibrosis (CF), but the interdependence of these phenotypes is poorly understood. We characterized S. aureus isolates from adult CF patients over several years. Of 18 S. aureus-positive patients (58%), 13 (72%) were positive for SCVs. Characterization included genotyping, SCCmec types, auxotrophy, biofilm production, antibiotic susceptibilities and tolerance, and resistance acquisition rates. Whole-genome sequencing revealed that several patients were colonized with prototypical and SCV-related clones. Some clonal pairs showed acquisition of aminoglycoside resistance that was not explained by aminoglycoside-modifying enzymes, suggesting a mutation-based process. The characteristics of SCVs that could play a role in resistance acquisition were thus investigated further. For instance, SCV isolates produced more biofilm (p < 0.05) and showed a higher survival rate upon exposure to ciprofloxacin and vancomycin compared to their prototypic associated clones. SCVs also developed spontaneous rifampicin resistance mutations at a higher frequency. Accordingly, a laboratory-derived SCV (ΔhemB) acquired resistance to ciprofloxacin and gentamicin faster than its parent counterpart after serial passages in the presence of sub-inhibitory concentrations of antibiotics. These results suggest a role for SCVs in the establishment of persistent antibiotic-resistant clones in adult CF patients.
Keywords: MRSA; Staphylococcus aureus; antibiotic resistance; cystic fibrosis; persister cells; resistance mutation; small-colony variant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- The Canadian Cystic Fibrosis Registry Annual Report. In Cystic Fibrosis Canada [Internet] 2023. [(accessed on 1 May 2023)]. pp. 1–54. Available online: www.cysticfibrosis.ca.
-
- 2021 Annual Data Report—Cystic Fibrosis Foundation Patient Registry. 2022. [(accessed on 1 May 2023)]. Available online: https://www.cff.org/medical-professionals/patient-registry.
-
- Sagel S.D., Gibson R.L., Emerson J., McNamara S., Burns J.L., Wagener J.S., Ramsey B.W. Inhaled Tobramycin in Young Children Study Group; Cystic Fibrosis Foundation Therapeutics Development Network. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J. Pediatr. 2009;154:183–188. doi: 10.1016/j.jpeds.2008.08.001. - DOI - PMC - PubMed
-
- Cogen J.D., Hall M., Faino A.V., Ambroggio L., Blaschke A.J., Brogan T.V., Cotter J.M., Gibson R.L., Grijalva C.G., Hersh A.L., et al. Antibiotics and outcomes of CF pulmonary exacerbations in children infected with MRSA and Pseudomonas aeruginosa. J. Cyst. Fibros. 2022;22:313–319. doi: 10.1016/j.jcf.2022.08.001. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

