Co-Production of Poly(3-hydroxybutyrate) and Gluconic Acid from Glucose by Halomonas elongata
- PMID: 37370574
- PMCID: PMC10294990
- DOI: 10.3390/bioengineering10060643
Co-Production of Poly(3-hydroxybutyrate) and Gluconic Acid from Glucose by Halomonas elongata
Abstract
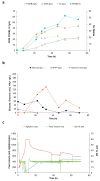
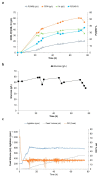
Polyhydroxyalkanoates (PHA) are biopolyesters regarded as an attractive alternative to petroleum-derived plastics. Nitrogen limitation and phosphate limitation in glucose cultivations were evaluated for poly(3-hydroxybutyrate) (P(3HB)) production by Halomonas elongata 1H9T, a moderate halophilic strain. Co-production of P(3HB) and gluconic acid was observed in fed-batch glucose cultivations under nitrogen limiting conditions. A maximum P(3HB) accumulation of 53.0% (w/w) and a maximum co-production of 133 g/L of gluconic acid were attained. Fed-batch glucose cultivation under phosphate limiting conditions resulted in a P(3HB) accumulation of only 33.3% (w/w) and no gluconic acid production. As gluconic acid is a valuable organic acid with extensive applications in several industries, this work presents an interesting approach for the future development of an industrial process aiming at the co-production of an intracellular biopolymer, P(3HB), and a value-added extracellular product, gluconic acid.
Keywords: Halomonas elongata; gluconic acid; halophiles; polyhydroxyalkanoates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cristea A., Baricz A., Leopold N., Floare C.G., Borodi G., Kacso I., Tripon S., Bulzu P.A., Andrei A.-Ș., Cadar O., et al. Polyhydroxybutyrate Production by an Extremely Halotolerant Halomonas elongata Strain Isolated from the Hypersaline Meromictic Fără Fund Lake (Transylvanian Basin, Romania) J. Appl. Microbiol. 2018;125:1343–1357. doi: 10.1111/jam.14029. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

