Enhancement of Antibacterial Properties, Surface Morphology and In Vitro Bioactivity of Hydroxyapatite-Zinc Oxide Nanocomposite Coating by Electrophoretic Deposition Technique
- PMID: 37370624
- PMCID: PMC10295605
- DOI: 10.3390/bioengineering10060693
Enhancement of Antibacterial Properties, Surface Morphology and In Vitro Bioactivity of Hydroxyapatite-Zinc Oxide Nanocomposite Coating by Electrophoretic Deposition Technique
Abstract
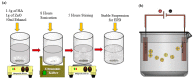
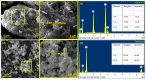
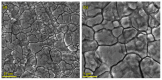
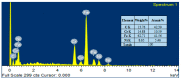
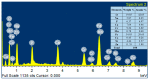
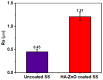
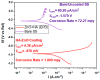
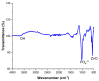
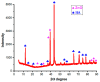
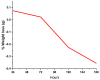
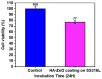
To develop medical-grade stainless-steel 316L implants that are biocompatible, non-toxic and antibacterial, such implants need to be coated with biomaterials to meet the current demanding properties of biomedical materials. Hydroxyapatite (HA) is commonly used as a bone implant coating due to its excellent biocompatible properties. Zinc oxide (ZnO) nanoparticles are added to HA to increase its antibacterial and cohesion properties. The specimens were made of a stainless-steel grade 316 substrate coated with HA-ZnO using the electrophoretic deposition technique (EPD), and were subsequently characterized using scanning electron microscopy (SEM), energy dispersive X-ray (EDX), stylus profilometry, electrochemical corrosion testing and Fourier transform infrared (FTIR) spectroscopy. Additionally, cross-hatch tests, cell viability assays, antibacterial assessment and in vitro activity tests in simulated body fluid (SBF) were performed. The results showed that the HA-ZnO coating was uniform and resistant to corrosion in an acceptable range. FTIR confirmed the presence of HA-ZnO compositions, and the in vitro response and adhesion were in accordance with standard requirements for biomedical materials. Cell viability confirmed the viability of cells in an acceptable range (>70%). In addition, the antibacterial activity of ZnO was confirmed on Staphylococcus aureus. Thus, the HA-ZnO samples are recommended for biomedical applications.
Keywords: electrophoretic deposition; hydroxyapatite; invitro study; nanocomposites; surface morphology; zinc oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Wright M., Uddin A. Organic—inorganic hybrid solar cells: A comparative review. Sol. Energy Mater. Sol. Cells. 2012;107:87–111. doi: 10.1016/j.solmat.2012.07.006. - DOI
-
- Schatkoski V.M., do Amaral Montanheiro T.L., de Menezes B.R.C., Pereira R.M., Rodrigues K.F., Ribas R.G., da Silva D.M., Thim G.P. Current advances concerning the most cited metal ions doped bioceramics and silicate-based bioactive glasses for bone tissue engineering. Ceram. Int. 2021;47:2999–3012. doi: 10.1016/j.ceramint.2020.09.213. - DOI
LinkOut - more resources
Full Text Sources

