Cancer Cell-Intrinsic Alterations Associated with an Immunosuppressive Tumor Microenvironment and Resistance to Immunotherapy in Lung Cancer
- PMID: 37370686
- PMCID: PMC10295869
- DOI: 10.3390/cancers15123076
Cancer Cell-Intrinsic Alterations Associated with an Immunosuppressive Tumor Microenvironment and Resistance to Immunotherapy in Lung Cancer
Abstract
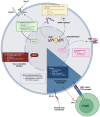
Despite the great clinical success of immunotherapy in lung cancer patients, only a small percentage of them (<40%) will benefit from this therapy alone or combined with other strategies. Cancer cell-intrinsic and cell-extrinsic mechanisms have been associated with a lack of response to immunotherapy. The present study is focused on cancer cell-intrinsic genetic, epigenetic, transcriptomic and metabolic alterations that reshape the tumor microenvironment (TME) and determine response or refractoriness to immune checkpoint inhibitors (ICIs). Mutations in KRAS, SKT11(LKB1), KEAP1 and TP53 and co-mutations of these genes are the main determinants of ICI response in non-small-cell lung cancer (NSCLC) patients. Recent insights into metabolic changes in cancer cells that impose restrictions on cytotoxic T cells and the efficacy of ICIs indicate that targeting such metabolic restrictions may favor therapeutic responses. Other emerging pathways for therapeutic interventions include epigenetic modulators and DNA damage repair (DDR) pathways, especially in small-cell lung cancer (SCLC). Therefore, the many potential pathways for enhancing the effect of ICIs suggest that, in a few years, we will have much more personalized medicine for lung cancer patients treated with immunotherapy. Such strategies could include vaccines and chimeric antigen receptor (CAR) cells.
Keywords: cancer cell-intrinsic; gene mutations; immunotherapy resistance; lung cancer.
Conflict of interest statement
L.M. Montuenga reports AstraZeneca and Bristol-Myers Squibb research grants and the AstraZeneca speakers bureau. A. Calvo reports a grant from AstraZeneca and a grant from PharmaMar. The rest of the authors declare no conflict of interest.
Figures

References
-
- Travis W.D., Brambilla E., Nicholson A.G., Yatabe Y., Austin J.H.M., Beasley M.B., Chirieac L.R., Dacic S., Duhig E., Flieder D.B., et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances since the 2004 Classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

