CD28 and 41BB Costimulatory Domains Alone or in Combination Differentially Influence Cell Surface Dynamics and Organization of Chimeric Antigen Receptors and Early Activation of CAR T Cells
- PMID: 37370693
- PMCID: PMC10295952
- DOI: 10.3390/cancers15123081
CD28 and 41BB Costimulatory Domains Alone or in Combination Differentially Influence Cell Surface Dynamics and Organization of Chimeric Antigen Receptors and Early Activation of CAR T Cells
Abstract
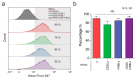
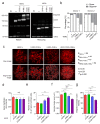
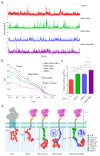
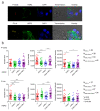
Chimeric antigen receptor (CAR)-modified T cells brought a paradigm shift in the treatment of chemotherapy-resistant lymphomas. Conversely, clinical experience with CAR T cells targeting solid tumors has been disheartening, indicating the necessity of their molecular-level optimization. While incorporating CD28 or 41BB costimulatory domains into CARs in addition to the CD3z signaling domain improved the long-term efficacy of T cell products, their influence on early tumor engagement has yet to be elucidated. We studied the antigen-independent self-association and membrane diffusion kinetics of first- (.z), second- (CD28.z, 41BB.z), and third- (CD28.41BB.z) generation HER2-specific CARs in the resting T cell membrane using super-resolution AiryScan microscopy and fluorescence correlation spectroscopy, in correlation with RoseTTAFold-based structure prediction and assessment of oligomerization in native Western blot. While .z and CD28.z CARs formed large, high-density submicron clusters of dimers, 41BB-containing CARs formed higher oligomers that assembled into smaller but more numerous membrane clusters. The first-, second-, and third-generation CARs showed progressively increasing lateral diffusion as the distance of their CD3z domain from the membrane plane increased. Confocal microscopy analysis of immunological synapses showed that both small clusters of highly mobile CD28.41BB.z and large clusters of less mobile .z CAR induced more efficient CD3ζ and pLck phosphorylation than CD28.z or 41BB.z CARs of intermediate mobility. However, electric cell-substrate impedance sensing revealed that the CD28.41BB.z CAR performs worst in sequential short-term elimination of adherent tumor cells, while the .z CAR is superior to all others. We conclude that the molecular structure, membrane organization, and mobility of CARs are critical design parameters that can predict the development of an effective immune synapse. Therefore, they need to be taken into account alongside the long-term biological effects of costimulatory domains to achieve an optimal therapeutic effect.
Keywords: cell therapy; chimeric antigen receptor (CAR); electric cell-substrate impedance sensing; fluorescence correlation spectroscopy (FCS); immunological synapse; immunotherapy; receptor clustering; receptor mobility; structure prediction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P., Carpenter R.O., Stetler-Stevenson M., Yang J.C., Phan G.Q., Hughes M.S., Sherry R.M., et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. - DOI - PMC - PubMed
-
- Ahmed N., Brawley V.S., Hegde M., Robertson C., Ghazi A., Gerken C., Liu E., Dakhova O., Ashoori A., Corder A., et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. - DOI - PMC - PubMed
-
- Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. - DOI - PMC - PubMed
-
- Ahmed N., Ratnayake M., Savoldo B., Perlaky L., Dotti G., Wels W.S., Bhattacharjee M.B., Gilbertson R.J., Shine H.D., Weiss H.L., et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–5964. doi: 10.1158/0008-5472.CAN-06-4309. - DOI - PubMed
Grants and funding
- OTKA K119690, K143771 and FK132773/National Research, Development and Innovation Office, Hungary
- GINOP-2.3.2-15-2016-00044/European Regional Development Fund
- To A.S./János Bolyai Research Scholarship
- ÚNKP-21-5-DE-482, ÚNKP-22-3-II/New National Excellence Program of the Ministry for Innovation and Technology
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

