Integrating Multi-Omics Analysis for Enhanced Diagnosis and Treatment of Glioblastoma: A Comprehensive Data-Driven Approach
- PMID: 37370767
- PMCID: PMC10296097
- DOI: 10.3390/cancers15123158
Integrating Multi-Omics Analysis for Enhanced Diagnosis and Treatment of Glioblastoma: A Comprehensive Data-Driven Approach
Abstract
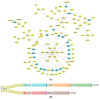
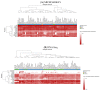
The most aggressive primary malignant brain tumor in adults is glioblastoma (GBM), which has poor overall survival (OS). There is a high relapse rate among patients with GBM despite maximally safe surgery, radiation therapy, temozolomide (TMZ), and aggressive treatment. Hence, there is an urgent and unmet clinical need for new approaches to managing GBM. The current study identified modules (MYC, EGFR, PIK3CA, SUZ12, and SPRK2) involved in GBM disease through the NeDRex plugin. Furthermore, hub genes were identified in a comprehensive interaction network containing 7560 proteins related to GBM disease and 3860 proteins associated with signaling pathways involved in GBM. By integrating the results of the analyses mentioned above and again performing centrality analysis, eleven key genes involved in GBM disease were identified. ProteomicsDB and Gliovis databases were used for determining the gene expression in normal and tumor brain tissue. The NetworkAnalyst and the mGWAS-Explorer tools identified miRNAs, SNPs, and metabolites associated with these 11 genes. Moreover, a literature review of recent studies revealed other lists of metabolites related to GBM disease. The enrichment analysis of identified genes, miRNAs, and metabolites associated with GBM disease was performed using ExpressAnalyst, miEAA, and MetaboAnalyst tools. Further investigation of metabolite roles in GBM was performed using pathway, joint pathway, and network analyses. The results of this study allowed us to identify 11 genes (UBC, HDAC1, CTNNB1, TRIM28, CSNK2A1, RBBP4, TP53, APP, DAB1, PINK1, and RELN), five miRNAs (hsa-mir-221-3p, hsa-mir-30a-5p, hsa-mir-15a-5p, hsa-mir-130a-3p, and hsa-let-7b-5p), six metabolites (HDL, N6-acetyl-L-lysine, cholesterol, formate, N, N-dimethylglycine/xylose, and X2. piperidinone) and 15 distinct signaling pathways that play an indispensable role in GBM disease development. The identified top genes, miRNAs, and metabolite signatures can be targeted to establish early diagnostic methods and plan personalized GBM treatment strategies.
Keywords: autophagy; biomarker selection; glioblastoma; inflammationomics; metabolomics; network analysis; pathway analysis; personalized therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Shojaei S., Koleini N., Samiei E., Aghaei M., Cole L.K., Alizadeh J., Islam M.I., Vosoughi A.R., Albokashy M., Butterfield Y., et al. Simvastatin increases temozolomide-induced cell death by targeting the fusion of autophagosomes and lysosomes. FEBS J. 2020;287:1005–1034. doi: 10.1111/febs.15069. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

