Effect of Mitotane on Male Gonadal Function
- PMID: 37370841
- PMCID: PMC10296642
- DOI: 10.3390/cancers15123234
Effect of Mitotane on Male Gonadal Function
Abstract
Background: Clinical evidence has shown frequent hypogonadism following mitotane (MTT) treatment in male patients with adrenocortical carcinoma. This study aimed to evaluate the impact of MTT on male gonadal function.
Methods: Morphological analysis of testes and testosterone assays were performed on adult CD1 MTT-treated and untreated mice. The expression of key genes involved in interstitial and tubular compartments was studied by real-time PCR. Moreover, quantitative and qualitative analysis of spermatozoa was performed.
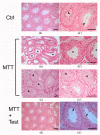
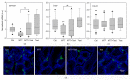
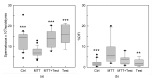
Results: Several degrees of damage to the testes and a significant testosterone reduction in MTT-treated mice were observed. A significant decline in 3βHsd1 and Insl3 mRNA expression in the interstitial compartment confirmed an impairment of androgen production. Fsh-R mRNA expression was unaffected by MTT, proving that Sertoli cells are not the drug's primary target. Sperm concentrations were significantly lower in MTT-treated animals. Moreover, the drug caused a significant increase in the percentage of spermatozoa with abnormal chromatin structures.
Conclusion: MTT negatively affects the male reproductive system, including changes in the morphology of testicular tissue and reductions in sperm concentration and quality.
Keywords: adrenocortical carcinoma; male gonadal function; mitotane; testis; testosterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fassnacht M., Assie G., Baudin E., Eisenhofer G., de la Fouchardiere C., Haak H.R., de Krijger R., Porpiglia F., Terzolo M., Berruti A. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:1476–1490. doi: 10.1016/j.annonc.2020.08.2099. - DOI - PubMed
-
- Stigliano A., Chiodini I., Giordano R., Faggiano A., Canu L., Della Casa S., Loli P., Luconi M., Mantero F., Terzolo M. Management of adrenocortical carcinoma: A consensus statement of the Italian Society of Endocrinology (SIE) J. Endocrinol. Investig. 2016;39:103–121. doi: 10.1007/s40618-015-0349-9. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

