Horizontal Transfer of Malignant Traits and the Involvement of Extracellular Vesicles in Metastasis
- PMID: 37371036
- PMCID: PMC10297028
- DOI: 10.3390/cells12121566
Horizontal Transfer of Malignant Traits and the Involvement of Extracellular Vesicles in Metastasis
Abstract
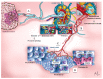
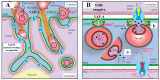
Metastases are responsible for the vast majority of cancer deaths, yet most therapeutic efforts have focused on targeting and interrupting tumor growth rather than impairing the metastatic process. Traditionally, cancer metastasis is attributed to the dissemination of neoplastic cells from the primary tumor to distant organs through blood and lymphatic circulation. A thorough understanding of the metastatic process is essential to develop new therapeutic strategies that improve cancer survival. Since Paget's original description of the "Seed and Soil" hypothesis over a hundred years ago, alternative theories and new players have been proposed. In particular, the role of extracellular vesicles (EVs) released by cancer cells and their uptake by neighboring cells or at distinct anatomical sites has been explored. Here, we will outline and discuss these alternative theories and emphasize the horizontal transfer of EV-associated biomolecules as a possibly major event leading to cell transformation and the induction of metastases. We will also highlight the recently discovered intracellular pathway used by EVs to deliver their cargoes into the nucleus of recipient cells, which is a potential target for novel anti-metastatic strategies.
Keywords: cancer; exosomes; extracellular vesicles; metastasis; microenvironment; nucleoplasmic reticulum; oncogene; tumor suppressor gene.
Conflict of interest statement
D.C. and A.L. declare conflicts of interest as the following patents are pending: US-2021-0353616-A1 (USA), EP3864409 (Europe), GB2575070 (UK) and GB2598624 (UK). All other authors declare no conflict of interest.
Figures



References
-
- Peinado H., Aleckovic M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., Garcia-Santos G., Ghajar C., et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

