The Multi-Faceted Nature of Renalase for Mitochondrial Dysfunction Improvement in Cardiac Disease
- PMID: 37371077
- PMCID: PMC10297141
- DOI: 10.3390/cells12121607
The Multi-Faceted Nature of Renalase for Mitochondrial Dysfunction Improvement in Cardiac Disease
Abstract
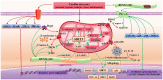
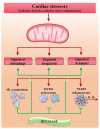
The cellular mechanisms and signaling network that guide the cardiac disease pathophysiology are inextricably intertwined, which explains the current scarcity of effective therapy and to date remains the greatest challenge in state-of-the-art cardiovascular medicine. Accordingly, a novel concept has emerged in which cardiomyocytes are the centerpiece of therapeutic targeting, with dysregulated mitochondria as a critical point of intervention. Mitochondrial dysfunction pluralism seeks a multi-faceted molecule, such as renalase, to simultaneously combat the pathophysiologic heterogeneity of mitochondria-induced cardiomyocyte injury. This review provides some original perspectives and, for the first time, discusses the functionality spectrum of renalase for mitochondrial dysfunction improvement within cardiac disease, including its ability to preserve mitochondrial integrity and dynamics by suppressing mitochondrial ΔΨm collapse; overall ATP content amelioration; a rise of mtDNA copy numbers; upregulation of mitochondrial genes involved in oxidative phosphorylation and cellular vitality promotion; mitochondrial fission inhibition; NAD+ supplementation; sirtuin upregulation; and anti-oxidant, anti-apoptotic, and anti-inflammatory traits. If verified that renalase, due to its multi-faceted nature, behaves like the "guardian of mitochondria" by thwarting pernicious mitochondrial dysfunction effects and exerting therapeutic potential to target mitochondrial abnormalities in failing hearts, it may provide large-scale benefits for cardiac disease patients, regardless of the underlying causes.
Keywords: MAPK; NAD+; apoptosis; cardiac disease; mitochondrial dysfunction; oxidative stress; renalase; sirtuins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
-
- Sanchez-Vinas A., Corral-Partearroyo C., Gil-Girbau M., Penarrubia-Maria M.T., Gallardo-Gonzalez C., Olmos-Palenzuela M.D., Aznar-Lou I., Serrano-Blanco A., Rubio-Valera M. Effectiveness and cost-effectiveness of an intervention to improve Initial Medication Adherence to treatments for cardiovascular diseases and diabetes in primary care: Study protocol for a pragmatic cluster randomised controlled trial and economic model (the IMA-cRCT study) BMC Prim. Care. 2022;23:170. - PMC - PubMed
-
- Brown D.A., Perry J.B., Allen M.E., Sabbah H.N., Stauffer B.L., Shaikh S.R., Cleland J.G., Colucci W.S., Butler J., Voors A.A., et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017;14:238–250. doi: 10.1038/nrcardio.2016.203. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

