Exploiting an Interleukin-15 Heterodimeric Agonist (N803) for Effective Immunotherapy of Solid Malignancies
- PMID: 37371081
- PMCID: PMC10297013
- DOI: 10.3390/cells12121611
Exploiting an Interleukin-15 Heterodimeric Agonist (N803) for Effective Immunotherapy of Solid Malignancies
Abstract
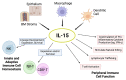
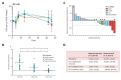
Identifying effective immunotherapies for solid tumors remains challenging despite the significant clinical responses observed in subsets of patients treated with immune checkpoint inhibitors. Interleukin-15 (IL-15) is a promising cytokine for the treatment of cancer as it stimulates NK and CD8+ lymphocytes. However, unfavorable pharmacokinetics and safety concerns render recombinant IL-15 (rIL-15) a less attractive modality. These shortcomings were addressed by the clinical development of heterodimeric IL-15 agonists, including N803. In preclinical tumor models, N803 elicited significant Th1 immune activation and tumor suppressive effects, primarily mediated by NK and CD8+ T lymphocytes. In addition, multiple clinical studies have demonstrated N803 to be safe for the treatment of cancer patients. The combination of N803 with the immune checkpoint inhibitor nivolumab demonstrated encouraging clinical responses in nivolumab-naïve and nivolumab-refractory patients with non-small cell lung cancer. In a recent Phase II/III clinical study, most Bacillus Calmette-Guerin (BCG)-refractory bladder cancer patients treated with N803 plus BCG experienced durable complete responses. Currently, N803 is being evaluated preclinically and clinically in combination with various agents, including chemotherapeutics, immune checkpoint inhibitors, vaccines, and other immuno-oncology agents. This report will review the mechanism(s) of action of N803 and how it relates to the preclinical and clinical studies of N803.
Keywords: Anktiva®; IL-15; N803; cancer immunotherapy; cytokine.
Conflict of interest statement
NIH authors declared no potential conflict of interest concerning the research, authorship, and/or publication of this article. PSS is an officer of ImmunityBio.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

